6.2 Reconstituting Medications
Learning Objectives
By the end of this chapter, the learner will be able to:
- define reconstitution, identify the correct amount of liquid to be added to dried medications, and identify the final concentration of medication.
Define Reconstitution, Identify the Correct Amount of Liquid to be Added to Dried Medications, and Identify the Final Concentration of Medication.
Many medications are packaged as a dried powder inside a vial or bottle. To administer these medications parenterally or as an oral liquid medication, the liquid must be added to the powder which then dissolves into the liquid. Instructions supplied with the medication will direct exactly how much liquid should be added to the container to give a specific concentration of medication. The instructions might be found on the container, on the packaging, on paper inside the package, or in information sent by the pharmacy. In addition, directions as to the specific type of liquid to be used will also be present, most often normal saline or sterile water. Careful reading of instructions and use of equipment is required to ensure the right amount of liquid is measured out and added to the container. Additional considerations for the safe preparation of medications such as proper labeling, avoidance of contamination, etc. will not be discussed in the context of this manual. Seek out additional information in a nursing skills textbook, such as Clinical procedures for safer patient care. by Doyle and McCutcheon, 2015.
Practice questions will focus on the following steps in reconstituting a medication:
- Identifying the amount and type of liquid to reconstitute the powdered medication with.
- Identifying the final concentration of medication.
- Calculating the correct volume of medication for a single dose.
Example 1: Reconstituting clindamycin
Refer to the images below of a bottle of clindamycin and two sides of the packaging to answer the questions in this example.
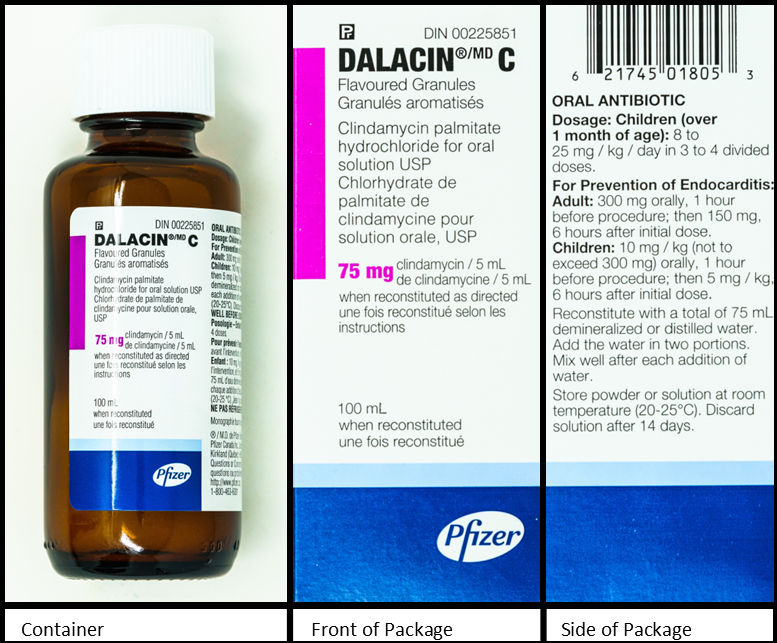
Questions:
- What type of liquid is used for reconstitution?
- How much of this liquid should be added to the container for reconstitution?
- What is the total volume in the container once reconstituted?
- What is the concentration of the reconstituted medication?
- What volume of medication would be poured from the bottle to give a dose of 90 mg?
Answers:
- demineralized or distilled water (this is found on the side of the package)
- 75 mL (this is found on the side of the package)
- 100 mL (this is found on the bottle and the front of the package)
- 75 mg/5 mL (this is found on the bottle and the front of the package)
- 6 mL
[latex]\text{mL}=\dfrac{\text{5 mL}}{\text{75 mg}}\times\text{90 mg}[/latex]
[latex]\text{mL}=6[/latex]
Exercises Part A: Reconstituting clarithromycin
Refer to the images below, of a bottle of clarithromycin and two sides of the packaging, to answer the questions in this example.
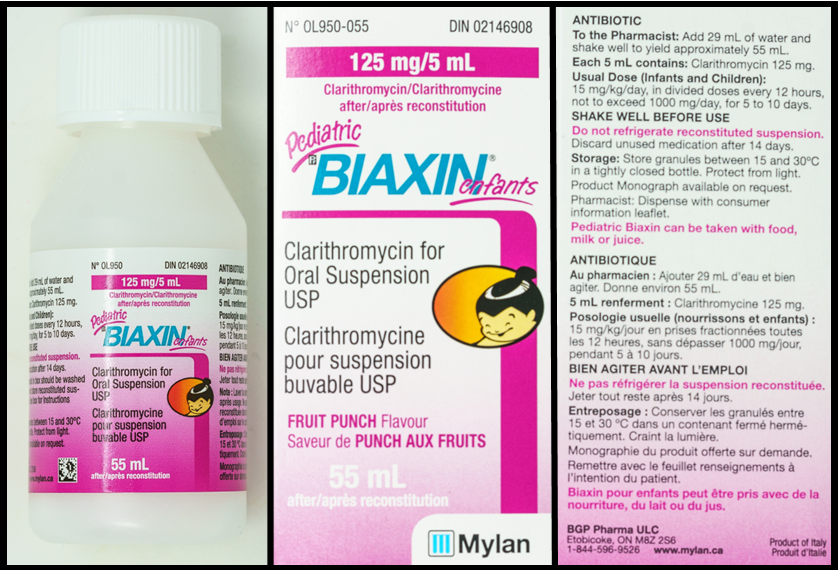
Questions:
- What type of liquid is used for reconstitution?
- How much of this liquid should be added to the bottle for reconstitution?
- What is the total volume in the container once reconstituted?
- What is the concentration of the reconstituted medication?
- What volume of medication would be poured from the bottle to give a dose of 50 mg?
Answers:
- water (this is found on the side of the package)
- 29 mL (this is found on the side of the package)
- 55 mL (this is found on the bottle and both sides of the package)
- 125 mg/5 mL (this is found on the bottle and both sides of the package)
- 2 mL
[latex]\text{mL}=\dfrac{\text{5 mL}}{\text{125 mg}}\times\text{50 mg}[/latex]
[latex]\text{mL}=2[/latex]
Exercises Part B: Reconstituting amoxicillin + clavulanate
Refer to the images below, of a bottle of amoxicillin + clavulanate and two sides of the packaging, to answer the questions in this example.
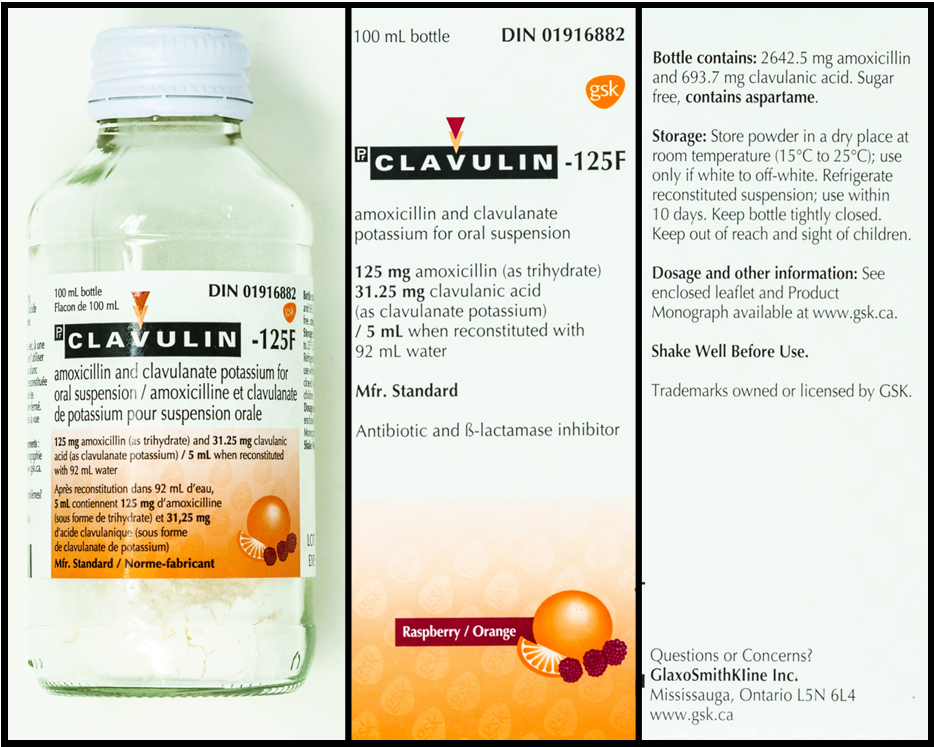
Questions:
- How much water should be added to the container for reconstitution?
- What is the total volume in the container once reconstituted?
- What is the concentration of the reconstituted medication?
Answers:
- 92 mL (this is found on the front of the bottle and the package)
- 100 mL (this is found on the bottle and the front of the package)
- 125 amoxicillin + 31.25 mg clavulanate/5 mL (this is found on the bottle and the front of the package)
Exercises Part C: Reconstituting azithromycin
Refer to the images below, of a bottle of azithromycin and two sides of the packaging, to answer the questions in this example.
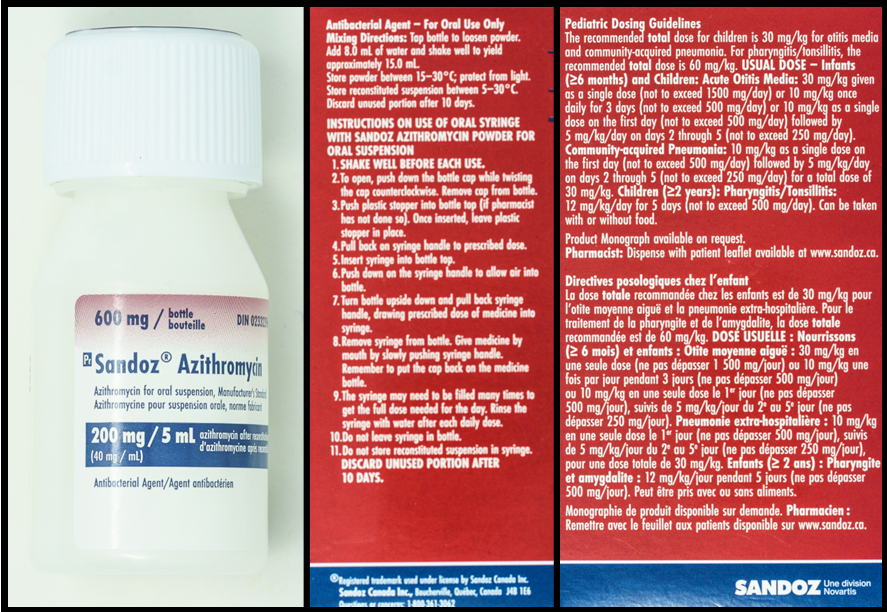
Questions:
- How much water should be added to the container for reconstitution?
- What is the total volume in the container once reconstituted?
- What is the concentration of the reconstituted medication?
- What volume of medication would be poured from the bottle to give a dose of 72 mg?
Answers:
- 8 mL (this is found on the skinny side of the package)
- 15 mL (this is found on the skinny side of the package)
- 200 mg/5 mL or 40 mg/mL (this is found on the bottle label)
- 1.8 mL
[latex]\text{mL}=\dfrac{\text{1 mL}}{\text{40 mg}}\times\text{72 mg}[/latex]
[latex]\text{mL}=1.8[/latex]
Exercises Part D: Reconstituting cefixime
Refer to the images below, of a bottle of cefixime and two sides of the packaging, to answer the questions in this example.
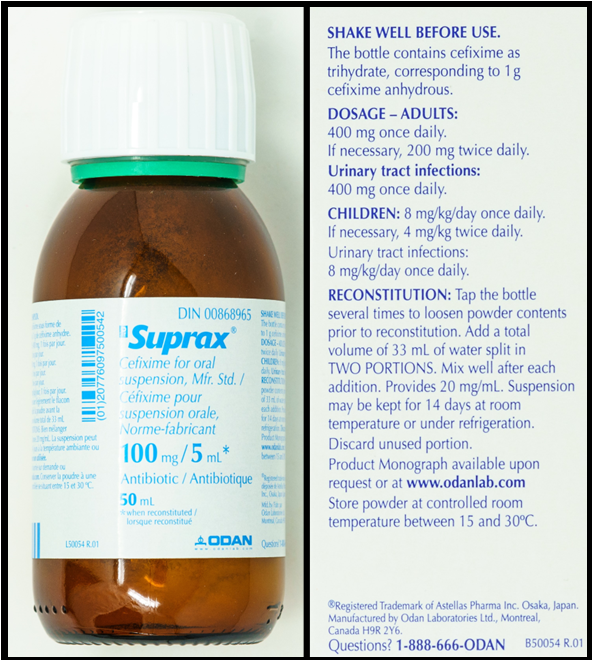
Questions:
- How much water should be added to the bottle for reconstitution?
- What is the total volume in the container once reconstituted?
- What is the concentration of the reconstituted medication?
- What volume of medication would be poured from the bottle to give a dose of 400 mg?
Answers:
- 33 mL total, split into two portions of 16 and 17 mL (this is found on the side of the package)
- 50 mL (this is found on the side of the package)
- 100 mg/5 mL (this is found on the bottle and the front of the package)
- 20 mL
[latex]\text{mL}=\dfrac{\text{5 mL}}{\text{100 mg}}\times\text{400 mg}[/latex]
[latex]\text{mL}=20[/latex]
Exercises Part E: Reconstituting cefprozil
Refer to the images below, of the front and side labels of a bottle of cefprozil, to answer the questions in this example.
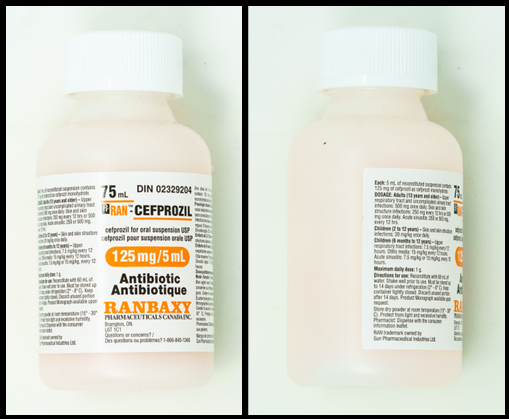
Questions:
- What volume of water should be added to the container for reconstitution?
- What is the total volume in the container once reconstituted?
- What is the concentration of the reconstituted medication?
- What volume of medication would be poured from the bottle to give a dose of 250 mg?
Answers:
- 60 mL (this is found on the side of the bottle)
- 75 mL (this is found on the front of the bottle)
- 125 mg/5 mL (this is found on the front and side of the bottle
- 10 mL
[latex]\text{mL}=\dfrac{\text{5 mL}}{\text{125 mg}}\times\text{250 mg}[/latex]
[latex]\text{mL}=10[/latex]
Exercises Part F: Reconstituting cephalexin
Refer to the images below, of the front and side labels of a bottle of cephalexin to answer the questions in this example.
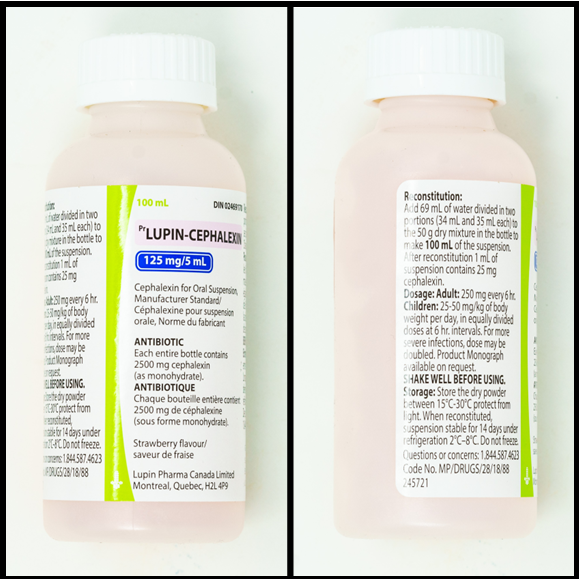
Questions:
- What volume of water should be added in the first step of mixing?
- What is the concentration of the reconstituted medication?
- What volume of medication would be poured from the bottle to give a dose of 87.5 mg?
Answers:
- 34 mL (this is found on the side of the bottle)
- 125 mg/5 mL (this is found on the front of the bottle) or 25 mg/mL (side of bottle)
- 3.5 mL
[latex]\text{mL}=\dfrac{\text{1 mL}}{\text{25 mg}}\times\text{87.5 mg}[/latex]
[latex]\text{mL}=3.5[/latex]
Transcribed Labels
- Front of label:
- Dalacin C. Flavoured granules.
- Clindamycin palmitate hydrochloride for oral solution USP.
- 75 mg clindamycin per 5 mL when reconstituted as directed.
- 100 mL when reconstituted.
- Side of label:
- Oral antibiotic.
- Dosage: Children (over 1 month of age): 8 to 25 mg per kg per day in 3 to 4 divided doses.
- For prevention of endocarditis:
- Adult: 300 mg orally, 1 hour before the procedure; then 150 mg, 6 hours after the initial dose.
- Children: 10 mg per kg (not to exceed 300 mg) orally, 1 hour before the procedure; then 5 mg per kg 6 hours after the initial dose.
- Reconstitute with a total of 75 mL of demineralized or distilled water. Add water in two potions. Mix well after each addition of water.
- Store power or solution at room temperature (20-25°C). Discard the solution after 14 days.
Click here to return to Example 1
Exercises Part A: Clarithromycin label
- Front of label:
- Pediatric Biaxin.
- 125 mg per 5 mL.
- Clarithromycin for oral suspension USP.
- Fruit punch flavour.
- 55 mL after reconstitution.
- Side of label:
- Antibiotic
- To the Pharmacist: Add 29 mL of water and shake well to yield approximately 55 mL.
- Each 5 mL contains Clarithromycin 125 mg.
- Usual dose (infants and children): 15 mg per kg per day, in divided doses every 12 hours, not to exceed 1000 mg per day for 5 to 10 days.
- Shake well before use.
- Do not refrigerate reconstituted suspension. Discard unused medication after 14 days.
- Storage: Store granules between 15 and 30°C in a tightly closed bottle. Protect from light.
Click here to return to Exercise A
Exercises Part B: Amoxicillin + clavulanate label
- Front of label:
- Clavulin. Amoxicillin and clavulanate potassium for oral suspension.
- 100 mL bottle.
- 125 mg amoxicillin (as trihydrate) and 31.25 mg clavulanic acid (as clavulanate potassium) per 5 mL when reconstituted with 92 mL water.
- Side of label:
- Bottle contains 2642.5 mg amoxicillin and 693.7 mg clavulanic acid. Sugar-free, and contains aspartame.
- Storage: Store powder in a dry place at room temperature (15-25°C); use only if white to off-white. Refrigerate reconstituted suspension; use within 10 days. Keep the bottle tightly closed. Keep out of reach and sight of children.
- Dosage and other information: See the enclosed leaflet and product monograph available at www.gsk.ca.
- Shake well before use.
Click here to return to Exercise B
Exercises Part C: Azithromycin label
- Front of label
- Sandoz® Azithromycin
- 600 mg per bottle
- Azithromycin for oral suspension
- 200 mg per 5 mL (40 mg per mL)
- Antibacterial agent
- Side 1 of the label:
- Antibacterial agent - for oral use only.
- Mixing directions: Top bottle to loosen powder. Add 8.0 mL of water and shake well to yield approximately 15.0 mL.
- Store powder between 15-30°C. Protect from light. Store reconstituted suspension between 5-30°C. Discard unused portion after 10 days.
- INSTRUCTIONS ON USE OF ORAL SYRINGE WITH SANDOZ AZITHROMYCIN POWDER FOR ORAL SUSPENSION.
- SHAKE WELL BEFORE EACH USE.
- To open, push down the bottle cap while twisting the cap counterclockwise. Remove the cap from the bottle.
- Push plastic stopper into bottle top (if the pharmacist has not done so). Once inserted, leave the plastic stopper in place.
- Pull back on the syringe handle to the prescribed dose.
- Insert the syringe into the bottle top.
- Push down the syringe handle to allow air into the bottle.
- Turn the bottle upside down and pull back the syringe handle, drawing the prescribed dose of medicine into the syringe.
- Remove the syringe from the bottle. Give medicine by mouth by slowly pushing the syringe handle. Remember to put the cap back on the medicine bottle.
- The syringe may need to be filled many times to get the full dose needed for the day. Rinse the syringe with water and have each daily dose.
- Do not leave the syringe in the bottle.
- Do not store reconstituted suspension in a syringe. DISCARD UNUSED PORTION AFTER 10 DAYS.
- Side 2 of the label: Pediatric dosing guidelines:
- The recommended total dose for children is 30 mg per kg for otitis media and community-acquired pneumonia. For pharyngitis/tonsillitis, the recommended total dose is 60 mg per kg.
- Usual dose - infants (≥6 months) and children: Acute otitis media: 30 mg per kg given as a single dose (not to exceed 1500 mg per day) or 10 mg per kg once daily for 3 days (not to exceed 500 mg per day) or 10 mg per kg as a single dose on the first day (not to exceed 500 mg per day) followed by 5 mg per kg per day on days 2 through 5 (not to exceed 250 mg per day).
- Community-acquired pneumonia: 10 mg per kg as a single dose on the first day (not to exceed 500 mg per day) followed by 5 mg per kg per day on days 2 through 5 (not to exceed 250 mg per day) for a total dose of 30 mg per kg.
- Children (≥2 years): Pharyngitis/tonsillitis: 12 mg per kg per day for 5 days (not to exceed 500 mg per day). Can be taken with or without food.
Click here to return to Exercise C
Exercises Part D: Cefixime label
- Front of label
- Suprax. Cefixime for oral suspension, Mfr. Std.
- 100 mg per 5 mL when reconstituted
- Antibiotic
- 50 mL
- Side of label:
- Shake well before use.
- The bottle contains cefixime as trihydrate, corresponding to 1 g cefixime anhydrous.
- Dosage - Adults: 400 mg once daily. If necessary, 200 mg twice daily. Urinary tract infections: 400 mg once daily.
- Children: 8 mg per kg per day once daily. If necessary, 4 mg per kg twice daily. Urinary tract infections: 8 mg per kg per day once daily.
- Reconstitution: Tap the bottle several times to loosen the powder contents before reconstitution. Add a total volume of 33 mL of water split in TWO POTIONS. Mix well after each addition. Provides 20 mg per mL.
- Suspension may be kept for 14 days at room temperature or under refrigeration. Discard unused portion. Store powder at controlled room temperature between 15 and 30°C.
Click here to return to Exercise D
Exercises Part E: Cefprozil label
- Front of label:
- Cefprozil
- 75 mL
-
Cefprozil for oral suspension USP
-
125mg/5mL
-
Antibiotic
-
Ranbaxy pharmaceuticals
- Side of label:
-
Each: 5 mL of reconstituted suspension contains 125 mg of cefprozil.
-
Directions for use: Reconstitute with 54 mL of water. Shake well before use.
-
Click here to return to Exercise E
Exercises Part F: Cephalexin label
- Front of label:
- Lupin-Cephalexin
- 100 mL
- 125 mg per 5 mL.
- Cephalexin for Oral Suspension, manufacturer standard.
- Antibiotic.
- Each bottle contains 25000 mg cephalexin (as monohydrate).
- Strawberry flavour.
- Side of label:
- Reconstitution: Add 69 mL of water divided into two potions (34 mL and 35 mL each) to the 50 g dry mixture in the bottle to make 100 mL of the suspension. After reconstitution, 1 mL of suspension contains 25 mg cephalexin.
- Dosage - Adults: 250 mg every 6 hr.
- Children: 25-50 mg per kg of body weight per day in equally divided doses at 6-hour intervals. For more severe infections, the dose may be doubled.
- SHAKE WELL BEFORE USING.
- Storage: Store the dry powder between 15-30°C. Protect from light. When reconstituted, suspension is stable for 14 days under refrigeration 2-8°C. Do not freeze.
Click here to return to Exercise F
Referring to parenteral administration of medications, which is by any route outside of the GI tract.
A 0.9% mixture of sodium chloride in water.

