Experiment 8: Evaporator – Steam Economy
Purpose
The purpose of this experiment is to study the effect of the pressure of the heating steam upon the rate of evaporation of water and to determine the steam economy of the system.
Introduction
An evaporator is a unit operation that is typically used to concentrate a solution by evaporating some of the solvent. In most cases, the solvent is water. To achieve temperatures above the boiling point of the solvent, steam is often used for heating purposes. Evaporation can take place at atmospheric pressure or under vacuum. When vacuum is present, the temperature at which the solvent will boil and evaporate is significantly less than at atmospheric pressure, which makes evaporation easier and faster. The pressure of the steam used for evaporation greatly affects the rate of evaporation; the higher the steam pressure (hence higher the temperature) the more water can be evaporated over a given length of time. The amount of water that has evaporated can readily be calculated by a simple mass balance. It should be noted that when the evaporation process occurs, it is assumed that only water is evaporating – any solute is left in the concentrate.
While the evaporation process itself is quite simplistic, the evaporator is much more complex. A in-depth review of types of evaporators is provided at the Visual Encyclopedia of Chemical Engineering: Evaporators, (n.d.), https://encyclopedia.che.engin.umich.edu/Pages/SeparationsChemical/Evaporators/Evaporators.html. Typically, the solvent evaporated needs to be recovered so as to not cause condensation problems throughout the plant. Also, because steam is used, a number of precautions need to be taken to minimize the problems of overheating equipment. In our lab, condensers and steam traps help to reduce some of these hazards. Below is an image of the evaporator in E030 with key components pointed-out.
Figure 1: Evaporator in E030
The driving force for the evaporation process is the temperature difference, ΔT, between the heating steam, Tsteam, and the boiling solution, Tliquid:
ΔT = Tsteam – Tliquid
This temperature difference can be increased by increasing the heating steam pressure (hence, the steam temperature) and/or by reducing the temperature of the boiling solution by applying a vacuum. Both Tsteam and Tliquid can be calculated from steam tables once the pressure of the supplied steam or of the boiling solution are known. The course tables can be used or web-sites as https://www.engineeringtoolbox.com/boiling-point-water-d_926.html for pressures higher than atmospheric and https://www.engineeringtoolbox.com/water-evacuation-pressure-temperature-d_1686.html for pressures below atmospheric.
The evaporator in E030 has been automated and most functions are controlled through an “operator station”. The user-interface of the operator station is provided in the image below, where most user functions are explained.
Figure 2: Evaporator equipment Operator Station
Procedure
The experimental procedure for this experiment has not been recorded. To recall how the evaporator operates, please review the experimental procedure video for the evaporator experiment (mass balance) from the previous semester: https://www.macvideo.ca/media/Evaporator_unit_operation/1_k7a9ccuj
This semester, evaporation is done first at atmospheric pressure and then under vacuum. The rate of evaporation is measured by weighing the condensation produced in a given time.
Runs at atmospheric pressure
- Drain the evaporator and the condensate drum.
- From the operator station, turn on the (service) condenser water.
- Close all valves, except C and D as marked on the evaporator.
- Open the cold water valve to fill the evaporator with water. The evaporator is full when the water level is near the top of the sight glass on the vapour-liquid separator.
- Open the main steam valve at the operator station and then adjust the steam pressure to 20 psig. This pressure will be constant throughout the run.
- Start timing the condensate collected when the solution has started boiling. Boiling can be assessed two ways:
- A constant solution temperature inside the evaporator. That temperature should be close to 100°C, but not exactly there.
- Water dripping from the condensate drum (provided that the solenoid valve above the drum is open)
- After 10 minutes, or when the water level has fallen to near the bottom of the separator sight glass, whichever comes first, turn off the steam.
- Collect the condensate in a weighed bucket
- Collect the “concentrated product” (i.e. the remaining water in the evaporator’s side)
- Do one more run at a different heating steam pressure.
Run under vacuum
- You can set the evaporator under vacuum by turning on the after-condenser cooling water and then opening the after-condenser steam valve at the operator’s station. When this valve is open, a vacuum of approximately 5 in Hg can be applied to the system. Do that.
- To fill the evaporator:
- Use a big tank and fill it up with approximately 30 liters of water (use the scale to measure exactly how much water you added to the tank).
- Since the system is under vacuum, the outlet hose can be used to suck the water into the evaporator. Do that and fill the evaporator with enough water (near to the top of the sight glass).
- Close the suction hose valve and weigh the tank with any remaining water.
- The difference between the weight of the tank before and after gives the amount of water into the evaporator.
- Turn on the heating steam and adjust to 25 psi. After-condenser steam and heating steam come from the same pipe so adjust both as necessary to keep the vacuum constant and the heating steam pressure constant.
- Complete the run as before, but note that:
- You will probably see no condensate flow as it all gets sucked into the vacuum line.
- Since there will be no condensate flow, assess the onset of boiling by monitoring the water’s temperature.
- Keep the process running for 10 minutes from the onset of boiling.
- At the end of the run, shut the vacuum off and collect and weigh the water remaining into the evaporator.
Report
Consult the steam economy calculation notes below.
- Calculate the evaporation rate for all runs.
- The evaporation rate is (kg water evaporated)/(evaporation time).
- Plot the evaporation rate versus ΔT (temperature difference between steam and boiling water) for each run (one point for each run; put all three points on the same graph). Comment on the relationship between evaporation rate and ΔT.
- To calculate ΔT consider as steam temperature the boiling point of water at the steam pressure and as the boiling water temperature the boiling point of water at the system’s pressure.
- Calculate the steam economy (see notes below) for all runs and comment on the results.
Steam Economy Calculation
In an evaporator, when the water boils and converts to steam, there is the potential to use this steam in a secondary evaporator to further concentrate a solution. This set up is quite common in industry; even introducing a 3rd evaporator in series can be employed. The goal is to increase the steam economy of the evaporator system. The steam economy is defined as the ratio between the solvent evaporated vs. the steam introduced into the system. For a single evaporator, the steam economy is typically 60 – 80%; when 2 or 3 evaporators are in series, the steam economy can approach 200%.
(1) ![]()
Where:
- Z = mass of steam used
- E = mass of water evaporated
To calculate steam economy using equation (1) above, a mass balance and energy balance are required. Here the mass balance considers feed, product and water evaporated;
(2) ![]()
Where:
- F = mass of feed (water fed into the evaporator)
- P = mass of product (water left into the evaporator that did not evaporate)
- E = mass of water evaporated (collected as condensate)
Along with the mass balance, an energy balance must be done. This states that the energy provided by the steam is used for heating-up all the water to its boiling point and for evaporating E kilograms of water.
(3) ![]()
Here,
- ΔHsteam is the enthalpy (energy) provided by 1 kilogram of steam. It can be calculated as
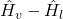 where the specific enthalpy values can be found in the course Tables at the steam’s pressure.
where the specific enthalpy values can be found in the course Tables at the steam’s pressure. - ΔHevaporation is the enthalpy (energy) required for evaporation of 1 kg of water. It can be calculated as
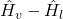 where the specific enthalpy values can be found in the course Tables at the system’s pressure.
where the specific enthalpy values can be found in the course Tables at the system’s pressure. - Cp specific heat capacity of liquid water. Assumed constant at 4.18 kJ/(kg °C).
- Tboiling boiling temperature of water. It depends on the system’s perssure
- Twater,in inflow temperature of water. It may be assumed constant at 16°C.
