Diffusion by Brownian Motion
Procedure
4.1 Sample Preparation
4.1.1 Mixing the Colloids
The bottle of paramagnetic colloids from the supplier is far too dense to use for this experiment. We need to dilute the colloids down to a volumetric fraction of about ![]() . The bottle from the supplier is 10% colloids by volume. I have already diluted that down by a factor of five.
. The bottle from the supplier is 10% colloids by volume. I have already diluted that down by a factor of five.
Use the micropipette to take 50 ![]() l of the diluted beads and add to about 1 ml of distilled water. You should be in the right ballpark of volumetric fraction to see individual chains of colloids when the magnetic field is applied. Shake your solution like there’s no tomorrow; we need to avoid the colloids clumping together.
l of the diluted beads and add to about 1 ml of distilled water. You should be in the right ballpark of volumetric fraction to see individual chains of colloids when the magnetic field is applied. Shake your solution like there’s no tomorrow; we need to avoid the colloids clumping together.
4.1.2 Preparing the Slide
- Put a clean microscope slide in the clamp in an orientation such that you can work with the top face of the slide.
- Use two pieces of double-sided tape to make a channel on the microscope slide that’s one or two millimeters across. Use a toothpick to push down the tape and make sure it is well-adhered to the slide.
- Stick a slide cover on top of the tape, over the channel, as shown in Figure 2. Use the toothpick to make sure that the slide cover is well adhered to the tape.
- Use the micropipette to add just a few droplets to the channel. You need to add enough of the colloid solution that the channel is filled, but not so much that it’s pouring out the other side.
- Use the toothpick to apply just a small amount of vacuum grease to the front and back of the channel, thereby locking in the colloid solution. Don’t make too much of a mess with the vacuum grease. A small amount of grease, applied well, is the best way to prevent your solution from leaking out halfway through your experiment.
- Put your slide under the microscope and, using the 40x objective, see if you can spot the colloids jiggling around all Brownian-like. When trying to view the beads, move the slide as close to the objective as you can while being sure to not touch the objective, then move the slide away using the microscope controls until the colloids come into view.
Figure 2: How to put together the slide, coverplate, and two-sided tape to make a channel for your colloid solution.
4.2 Setting Up the Initial Condition
- If you can see your colloids moving around in a Brownian fashion, then please continue on. Try to see if the colloids are clumping together or not. Talk to the TA or technician if you notice significant clumping, also called aggregation.
- The reference uses a Helmholtz coil to generate a magnetic field. I couldn’t quite figure out how to mount an electromagnetic near the objective of this microscope, so you’ll be using a bar magnet.
- Hold the bar magnet near the microscope slide. Watch on the screen as the colloids align themselves into long chains along the magnetic field lines. Is the width of the chain at this
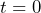 equal to zero? Consider that in your data fitting.
equal to zero? Consider that in your data fitting. - Try to hold the magnet in such a way that the chains are vertical on the screen. The analysis is still possible if the chains are at some angle, but it’s not as simple.
- Also note that the depth of the sample is non-zero, meaning that the chain can extend into and out of the plane that the microscope can view. Adjust the position of the slide stand to get the best view possible. Try to avoid resting your hand on the stage, since that will push it down, and could put the colloids out of view.
4.3 Video Capture
- Once you know that you can set your sample up for a measurement, click the capture button or hit F9. There are some menu items to click through, many of which aren’t necessary. EXCEPT FOR ONE!!! SAVE YOUR VIDEO AS AN AVI FILE!! Once you input the file name, but before you hit ‘finish’, use the bar magnet to set up your sample into nice straight lines. Click ‘finish’, then wait a second to know that the video has definitely started.
- Pull the bar magnet away from the sample without changing its angle, which would change the orientation of the chains.
- Let the video capture run until the paramagnetic colloids have diffused to the point that you can’t really define any chains of them anymore, then stop the capture.
- Check that you can load your video into imageJ and look at individual frames and have timestamps for them all.
- Do a few trials to try and capture the nicest diffusion possible. Sometimes the colloid chains are too close together, making analysis difficult. You may decide to make a new sample with a different concentration.
4.4 Calibration
- Your measurements up until this point have been in units of pixels, which aren’t actually useful. We need to calibrate our images.
- Put the calibration slide under the microscope and focus on to one of the structures with a labelled distance scale. Make sure it’s as well-focused as possible. Save an image.
- When you finish your analysis, you will be able to use this calibration as a final step to convert your results from pixels to meters.

