Plasma Physics
Experimental Apparatus
The apparatus you will be using is shown in Figure 5. As in Figure 1, the cathode is on the left and the anode is on the right. The Langmuir probe is mounted on the bottom of the Pyrex cross and the isolation valve, gas valve, and pressure sensor are all connected to the aluminum cross at the top of the Pyrex cross. The long tube on the left of the Pyrex cross can be removed, which you will need to do for the quantitative measurements. The longer tube is great for demonstrating the qualitative features of the plasma, but can make the quantitative stuff harder to measure precisely.
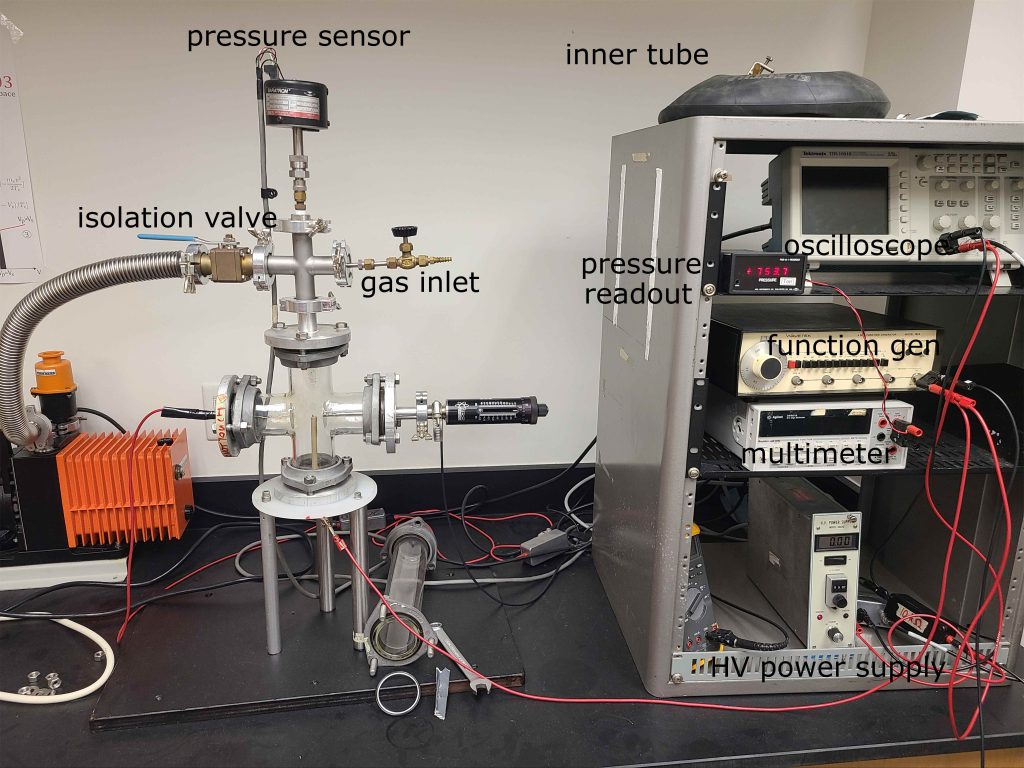
Figure 5: The DC discharge apparatus, sans plasma.
3.2 Electronics
The rack to the left of the plasma chamber houses the electronics that we’ll need for these measurements. There is a high voltage power supply that is connected to the anode and cathode to generate the potential difference in the plasma tube. A digital multimeter is used to precisely determine the applied voltage. A function generator is used to provide the retarding voltage to the Langmuir probe and an oscilloscope is used to measure that retarding voltage and the resulting probe current (measured as the voltage drop across a 10 kΩ resistor). This way, both measurements are saved in a single scope trace, and can be saved to a USB drive to plot with the program of your choice (choose Python). Finally, the small black box in the middle displays the pressure. It has an analog output that can be connected to the digital multimeter to get a more precise determination of the vacuum pressure than what is shown on the display. The pressure display has a zero-adjust so that the user can account for small drifts in the measured value.
3.2 Langmuir Probe
The Langmuir probe is just a piece of conductor; really nothing special (though sophisticated Langmuir probe assemblies do exist and are very useful). This apparatus uses a length of tungsten wire as the Langmuir probe. Most of the wire is shielded by a ceramic insulator. The exposed tungsten has a diameter of 1.65 mm and a length of 3.00 mm. Precise measurements of the surface area of the probe are necessary to determine the saturated electron current.
3.3 Vacuum System
The first principle in vacuum technology is that vacuum pumps don’t suck! They create a space of relatively low pressure. Gas molecules in the vacuum chamber fill this space, and are removed from the vacuum chamber by the motion of the pump.
A schematic of a rotary vane pump is shown in Figure 6. As the rotor turns, gas flows into the large section of the working chamber until it is sealed off by the second vane. The enclosed gas is then compressed until the outlet valve opens against atmospheric pressure.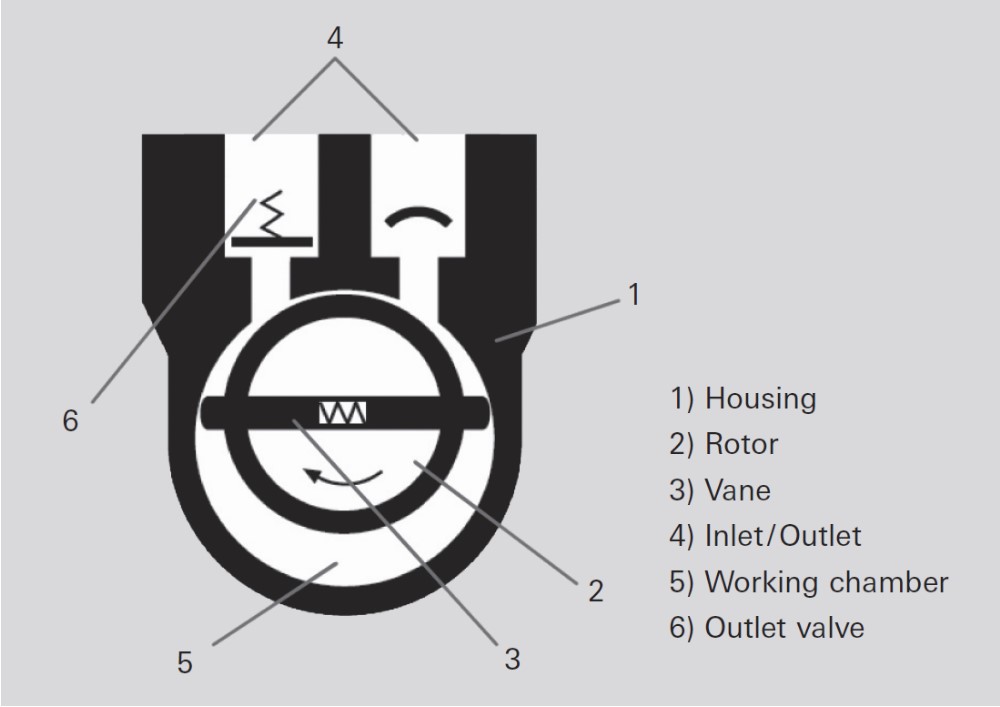
Figure 6: Schematic diagram of a rotary vane pump. Figure taken from www.pfeiffer-vacuum.com.
The pump can evacuate the system down to a pressure below 10 mTorr, which is essentially zero pressure as far as we are concerned here. You may notice that if you close the isolation valve, that the pressure will increase. If the increase is relatively slow, then it’s unlikely that there is a leak in the vacuum system. This increase in pressure is due to outgassing from the inner surface of the vacuum chamber. If the increase is fast, like more than one Torr in a minute, then there may be a leak. The most likely culprit is whatever vacuum seal you just changed.
Since we are using different species of gas in this experiment, we need a pressure sensor that isn’t species-sensitive. Most pressure sensors that work well at low pressures use convection between hot and cold surfaces to measure the pressure. But convection depends on the mass of the gas atoms between the two surfaces, so convection-based pressure sensors are often calibrated to nitrogen, and are almost useless in other gases. In this experiment, we use a capacitance-based pressure sensor. The capacitance between two surfaces is measured, and the change in capacitance as the surfaces deflect indicates the change in pressure.
