Thermal Conductivity of Gases
Procedure
4.1 Connecting Sensors
- The temperature sensor is already connected to the thermocouple and plugged into the breadboard (as in Figure 3). Open the Arduino program to see which pins from the temperature sensor connect to which digital pins of the Arduino and connect them accordingly. If you’ve never used a breadboard like this and you’re feeling confused, ask your TA about connecting pins.
- Now we supply power to the temperature sensor. Be very careful in this step that you are connecting to the correct voltage source and ground! The temperature sensor is connecting to the ‘rails’ of the breadboard. Connect the red rail (with red wire) to the 3.3 V output of the Arduino and the blue rail (with green wire) to the ground of the Arduino.
- Connect the voltage-divided output from the pressure sensor to the appropriate pins on the analog input side of the Arduino. Connect the red wire to the appropriate channel and the black wire to one of the ground channels.
- Upload the Arduino program.
- If the Arduino program uploaded successfully, then start ‘Spyder’ (a Python compiler) and run the thermal conductivity program. You should see some graphs and hopefully they start populating with data.
Figure 3: The Arduino, breadboard, temperature sensor, and pressure sensor output. Once everything is connected to the Arduino, the computer will be able to digitize all of this data so that it can be read and plotted by Python.
4.2 Thermal Conductivity as a Function of Power
- The apparatus will start out filled with air. Check the pressure reading on the Bourdon gauge at the back of the apparatus before you begin, just to be sure. If the chamber is under vacuum, set the valves to fill it with air from the atmosphere.
- Measure the temperature difference between the two cylinders with no power applied to the heater, and then with several powers up to 5 Watts. You will be fitting that data with a straight line, so be sure to take enough data that you are confident in your fit parameters. For each power, wait for the temperature to stabilize and record the measurement. It can take up to ten minutes for the temperature to stabilize. DO NOT WAIT FOREVER FOR THERMAL EQUILIBRIUM! You can take your data faster if you can accept a larger uncertainty in your temperature. You don’t need to save all of this data. You can write down the approximate difference and your estimate of the uncertainty based on what you see on the screen.
- Check that your temperature difference measurements are linear with applied power, otherwise either Equation (3) is not valid, or something about this experiment is seriously flawed. Determine
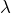 for air at atmospheric pressure and its uncertainty from the linear fit.
for air at atmospheric pressure and its uncertainty from the linear fit.
4.3 Thermal Conductivity as a Function of Pressure
- Keep applying power to the cylinders, restart the data acquisition software and turn on the vacuum pump. You’ll now see how
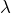 depends on pressure.
depends on pressure. - Slightly open the valve above the vacuum pump to slowly evacuate the chamber. Pump too slowly and the experiment will take an obnoxiously long amount of time; pump too quickly and you lose the assumption of thermal equilibrium between the cylinders. It’s okay to change pumping speed during the experiment, since you are only interested in temperature difference as a function of pressure, not time. Continue acquiring data until the chamber is evacuated, and the temperature difference vs pressure plot stops changing.
- You should see a plot that looks like a capital L: The pressure should decrease and the temperature difference should stay about the same, then the pressure should get low enough that the temperature difference increases. The intersection of the horizontal and vertical parts of this curve indicate the pressure at which the mean free path of the gas molecules is the same as the gap between cylinders.
- Save your data and turn off the power to the cylinder heater. Make sure you can open your data file before you leave the lab.
4.4 Thermal Conductivity of Various Gases
- Talk to the TA or lab tech about applying various gases to the vacuum chamber. We need to avoid uses the same gas at two different experiment stations at the same time
- You will use argon and helium, but start with argon since it’s easier to remove before switching gases. You’ll have to fill the vacuum chamber about halfway with gas, evacuate it with the pump, and refill the chamber with only one variety of gas. Be careful not to overfill the chamber, which would result in the glass chamber lifting off of the vacuum seal. You’ll get the same measurement for λ using even half of atmospheric pressure in the chamber.
- Once the chamber has a pure gas in it, apply power to the heater to measure λ for that gas. You only need one measurement for each gas – use the uncertainty in your measurement for air for these other gases, too.
- When your measurements are complete, turn off the heater, evacuate the chamber, then vent to atmosphere.

