Cloud Chamber
Introduction
2.1 The Cloud Chamber
The cloud chamber was the first device that allowed physicists to visualize individual particles and record data about individual nuclear events that, until then, could only be studied by averaging these events in bulk matter. Many particles were discovered using early cloud chambers, such as the positron and the muon. These particles were discovered using a Wilson cloud chamber, which uses a volume of air that has been super-saturated with water vapour. Droplets form along the path of any ionizing radiation that passes through the vapour, allowing the user to see a track where the particles had passed. The physics of this droplet formation is a topic unto itself, and is still actively researched [1].
In this experiment, we’ll use a slight variation on the Wilson cloud chamber, called the diffusion cloud chamber. The saturated volume of water vapour is replaced with a saturated volume of isopropyl alcohol. Instead of expanding the volume to super-saturate the air with water vapour, the bottom plate is cooled to at least -26◦ C to super-saturate the area just above the cold plate with isopropyl alcohol vapour. A schematic image of a cloud chamber is shown in Figure 1.
2.2 Detection Volume
Calculating the detector area for most modern detectors is very straight forward. For GM tubes and scintillating crystals, the user simply measures the surface area of the face of the detector (or looks in the user manual). The detection volume of the diffusion cloud chamber is not so easily determined. One needs to determine the height above the cold-plate that contains the super-saturated isopropyl vapour. One can determine the level of saturation of the air with isopropyl as a function of height from the cold-plate using the equations in Reference [3]. Solving the relevant equations and plotting them to find the overlapping region that is the height of the detector volume is an interesting and challenging exercise that you could do, but we’re going to focus more on radiation physics and statistics here, and just say that the height of the detector volume is about 4(1) mm (a 25% fractional uncertainty). This height depends on the temperature difference between the top and bottom of the detector, and so it will be slightly different depending on the ambient temperature and how well the bottom plate is being cooled.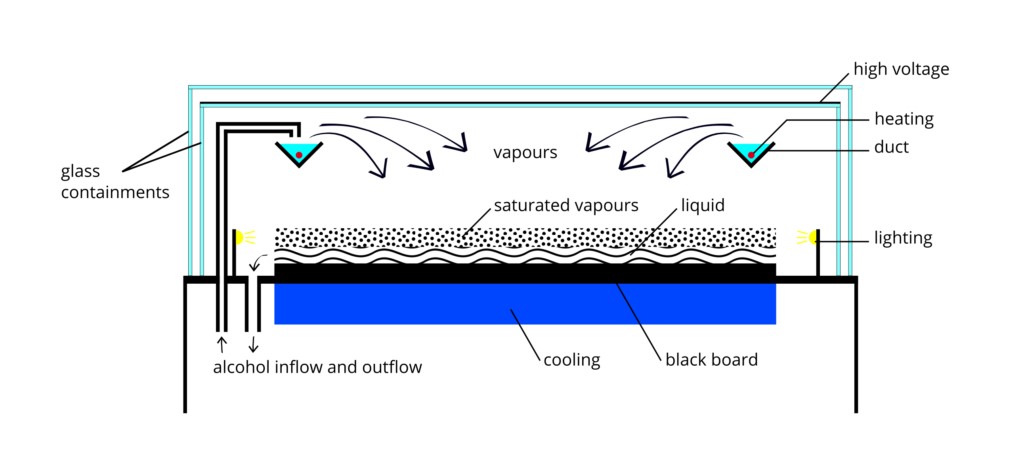
Figure 1: Schematic of a cloud chamber apparatus. The apparatus you will use has a much simpler design. Figure from Wikipedia.
|
radiation |
composition |
sufficient shielding |
|
alpha |
helium nucleus |
paper |
|
beta |
e− or e+ |
thin metal |
|
gamma |
photon |
thick lead |
|
neutron |
neutron |
water or concrete (lead does nothing) |
Table 1: Types of ionizing radiation and sufficient shielding
2.3 Alpha Radiation
The SI unit of radiation is the Becquerel. It’s pretty easy to understand: If you have a radioactive isotope that undergoes one decay event per second, then you have 1 Bq. Please note that 1 Bq is an incredibly small amount of radiation. For comparison, the radioactivity from a banana is about 15 Bq. Furthermore, not all radiation has the same hazard level. Four types of ionizing radiation are shown in Table 1.
Alpha radiation, which is what you will mostly be able to see in this cloud chamber, is usually the least dangerous of the four types of radiation. Alpha particles are relatively large, which means they are much slower than other particles of the same kinetic energy, and they have a +2 charge that makes them even more susceptible to the influence of electric charges in bulk matter. Alpha particles are easily stopped by even very-thin layers of matter such as human skin or paper, so alpha particles don’t pose a significant threat externally. Internally, however, alpha particles are a serious hazard, and one that’s present in many homes and buildings in the form of radon gas.
Radon (specifically ![]() ) is a colourless, odourless gas that results from the decay of
) is a colourless, odourless gas that results from the decay of ![]() . Radon can be dangerous when it builds up in poorly-ventilated basements and other structures. The radon has a chance to decay while in the lungs, exposing your internal organs to alpha radiation. In fact, radon is the leading causes of lung cancer in Canadian non-smokers.
. Radon can be dangerous when it builds up in poorly-ventilated basements and other structures. The radon has a chance to decay while in the lungs, exposing your internal organs to alpha radiation. In fact, radon is the leading causes of lung cancer in Canadian non-smokers.
