Gas Thermometry
Introduction
Matter, whether in gas, liquid or solid phase is composed of an extremely large number of microscopic particles, each having some amount of kinetic energy. This kinetic energy is divided among the degrees of freedom that the particle can access, which includes translational, vibrational, and rotational motion of the particles. While the velocity, rotation or vibration of a single molecule can be measured, it is impossible to directly measure the total kinetic energies of the particles which make up some macroscopic material. Temperature is a macroscopic concept which is used to measure or define the average microscopic kinetic energy of the particles in some material.
Since we can never measure the temperature of the object by summing up the kinetic energy of its constituent particles, temperature is always measured through some other proxy. For example, one can measure the expansion of the length of a column of mercury in a glass capillary, the bending of a bimetallic strip or a change in the electrical resistivity of a material or some other physical change. An instrument that measures the temperature of a material by measuring some other physical change is called a thermometer, and since the measurement is dependent on some physical change, each type of thermometer is limited to functioning over a limited temperature range.
2.1 The Gas Thermometer
In this experiment, three phase transitions to define our temperature scale:
- liquid-gaseous water (the boiling point of water, 100
 C)
C) - solid-liquid water (the melting point of ice, 0
 C)
C) - liquid-gaseous nitrogen (boiling point of liquid nitrogen −196
 C)
C)
We will use a gas thermometer to set up a temperature scale based on these reference points and attempt to measure another reference point – absolute zero, which is, by definition, outside the range of the measurement of such a thermometer.
You no doubt remember the Ideal Gas Law:
(1) ![]()
where ![]() is the measured pressure of a gas occupying a volume
is the measured pressure of a gas occupying a volume ![]() ,
, ![]() is the number of moles of the gas in the container,
is the number of moles of the gas in the container, ![]() (= 8.3145 J/mol·K) is the universal gas constant, and
(= 8.3145 J/mol·K) is the universal gas constant, and ![]() is the temperature measured on an absolute temperature scale (i.e. the Kelvin scale). Since our container is fixed and the gas is fixed (cannot leave or enter container and undergoes no chemical change):
is the temperature measured on an absolute temperature scale (i.e. the Kelvin scale). Since our container is fixed and the gas is fixed (cannot leave or enter container and undergoes no chemical change): ![]()
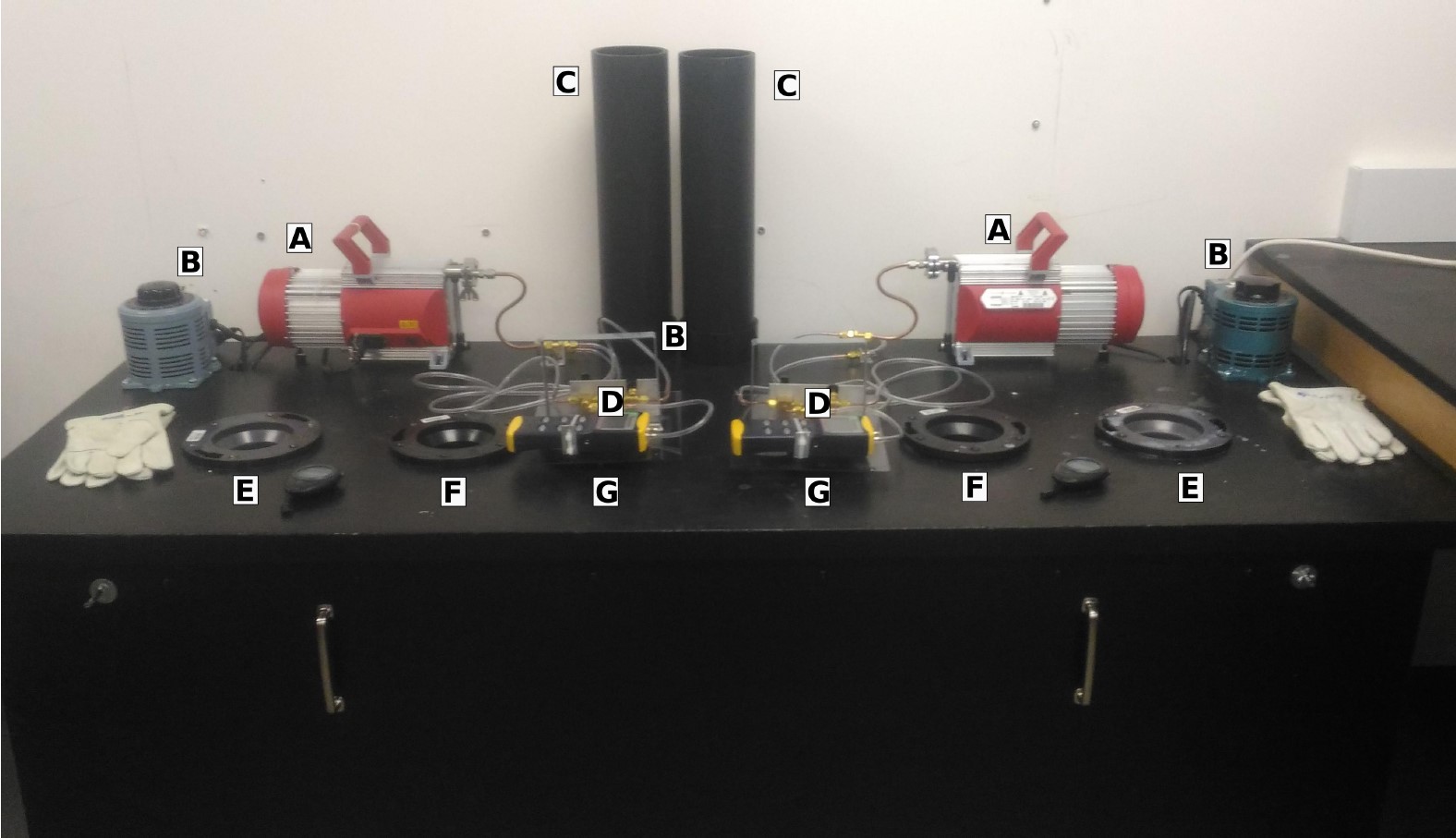
Figure 1: View of the main table for the Gas Thermometer setup showing both stations. Main parts are labelled: A) vacuum Pump B) variac for control of water boiling power C) temperature reserviour. D) thermometer unit with manometer and valving E) boiling water reservoir F) ice water reservoir G) liquid nitrogen reservoir
