Exercise 1: Surface Tension
A key topic in the study of fluids is surface tension. As the name implies, it refers to a force per unit length that is related to the surface of a fluid and is often described as the tendency of a fluid to minimize its surface area. Attraction between molecules at the surface leads to surface tension. To visualize this we can look at the schematic below:

Figure 1.1: Schematic of liquid molecules and the forces they feel at the surface compared to in the bulk of the liquid – this gives rise to surface tension.
Consider the following example: when you fill a container with liquid, do you notice anything about the shape of the liquid at the edges of the container? You may find that it is curved. This curvature is often referred to as a meniscus. A meniscus forms due to the attractive forces between molecules, as well as the attractive force between the fluid and the walls of the container. If the force between molecules is greater than between the fluid and the walls, then the fluid does not want to wet the walls of the container, forming a downwards curve (meniscus) which is shown in the test tube on the left-hand side in Figure 1.2. Conversely, if the force between the molecules and the wall is greater than the force between molecules, then the fluid will want to wet the walls of the container, forming an upwards curve (meniscus) as shown in the picture of the test tube on the right-hand side in Figure 1.2. In the case where the force between the molecules and the walls is greater, the liquid is pulled up the walls of the container against gravity, this phenomenon is known as capillary rise.

Figure 1.2: Two test tubes containing mercury (left) and water (right). Mercury forms a downwards meniscus, while water forms an upwards meniscus. Water likes the container walls, mercury does not.
Lastly, it is useful to define what a contact angle is for this lab. A contact angle is the angle where a fluid and solid surface meet as shown in Figure 1.3 below. In Figure 1.2 above, mercury is a case where the contact angle is greater than 90º, indicating low wettability – mercury does not like the container walls. Water is a case where the contact angle is less than 90º, indicating high wettability – water likes the container walls.
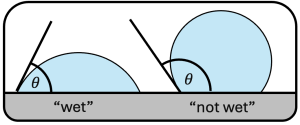
Figure 1.3 Two droplets on a surface with contact angles (θ). The droplet on the left is a fluid that ‘wets’ the surface (the contact angle is less than 90º), the droplet on the right is a fluid that ‘does not wet’ the surface (the contact angle is greater than 90º).
The following two experiments are more qualitative, so don’t worry about collecting data but make sure to make note of your observations.
For this experiment you will need to collect the following items:
- A container with water
- Paperclip
- A coin (aim to use a coin with a small radius, like a penny or a dime)
- A few drops of liquid soap
- A plastic dropper or equivalent (don’t worry if you don’t have one, you can use your finger as a dropper!)
Procedure 1:
Partially fill your container with water and float a paperclip on the water’s surface. You may notice that this will require a ‘light touch’. If not placed carefully, your paperclip will sink.
Exercise 1.1 (2 mark)
Submit a photograph of your floating paperclip and the student card(s) of the participating member(s) in this lab and upload to Crowdmark.
Secondly, provide an additional image of the student card(s) used in the previous photo. Here, the student name(s) and student number(s) must be legible. With your experimental set up in the background, you may take this image from a closer view to ensure the student card(s) is/are in full focus. Note: you do not need to be in the photo. If you are completing this lab with others virtually, you may provide a screenshot of your video call, with the student cards of all members visible. Your experimental set up must still be visible in the background.
Exercise 1.2 (2 marks)
The density of a paperclip is greater than water, yet it can still float on the surface if we place it gently enough. However, if you placed the paperclip just under the surface, it would sink. Explain in a few short sentences which forces are at play and how they differ between scenarios. Upload your answer to Crowdmark.
Procedure 2:
Ensure your coin is clean of any dirt or grime. Then, place your dry coin on a flat surface.
In the lab: Fill your water dropper with water and slowly add drops of water to the centre of your coin. Count how many drops you can add before the water spills over the side of the coin.
At home: Use your finger or something that can produce consistent sized water drops (e.g. clean out a food colouring dispenser, eyedropper, use a straw, poke a tiny hole in a plastic bag). Slowly add drops of water to the centre of your coin. Try your best to ensure the drops are of roughly the same volume. Count how many drops you can add before the water spills over the side of the coin.
Repeat the experiment but add 5-10 fewer drops than the previous answer. Take a picture. Then, add a drop of soap to the top of the coin – take note of what happened, you will be asked about it below.
Note: once the coin has soap on it the number of water drops it holds changes so if you need to repeat the experiment, clean the coin first.
Exercise 1.3 (2 mark)
Submit a photograph of your coin with water on top and the student card(s) of the participating member(s) in this lab and upload to Crowdmark.
Secondly, provide an additional image of the student card(s) used in the previous photo. Here, the student name(s) and student number(s) must be legible. With your experimental set up in the background, you may take this image from a closer view to ensure the student card(s) is/are in full focus. Note: you do not need to be in the photo. If you are completing this lab with others virtually, you may provide a screenshot of your video call, with the student cards of all members visible. Your experimental set up must still be visible in the background.
Exercise 1.4 (2 marks)
When you placed the drop of soap on the coin with water what happened? Provide an explanation for what you observed in a few short sentences. Hint: Refer to our discussion above about surface tension. Upload your response to Crowdmark.
Before you continue!
Before continuing, be sure you have completed Exercises 1.1-1.4 which will be graded and submitted through Crowdmark.

