10 Respiratory Diseases of Swine
| Course priority #1 | Course priority #2 | Course priority #3 | |
| Swine |
|
|
|
Inclusion body rhinitis
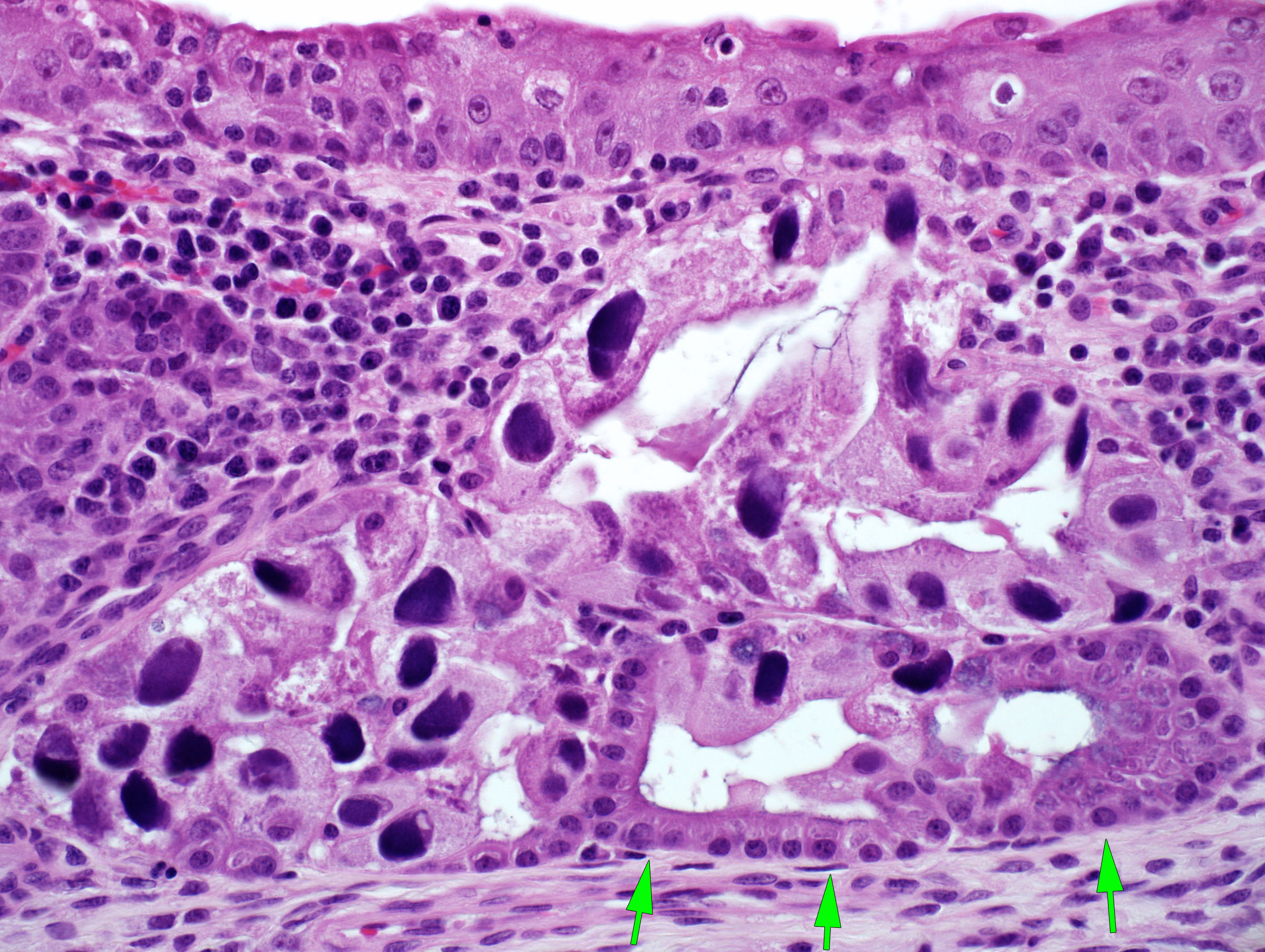
Caused by Porcine cytomegalovirus (Suid herpesvirus-2), a beta-herpesvirus. There is typically high morbidity but low mortality in baby pigs, with sneezing and nasal discharge. The diagnosis is based on the pathognomonic histologic lesions of epithelial enlargement (cytomegaly) and intranuclear inclusions. PCR testing is available. The infection may also cause stillbirths or abortion of mummified fetuses, and might play a role in the “post-weaning failure-to-thrive syndrome”.
Atrophic rhinitis
Atrophic rhinitis was once common but is now rare. It is caused by specific toxin-producing strains of Pasteurella multocida type D. Certain factors predispose to colonization of the mucosa with P. multocida, including infection with Bordetella bronchiseptica, and ammonia and dusts in the environment. Pasteurella multocida cytotoxin elicits resorption and atrophy of turbinate bone by osteoclasts. Clinical signs are sneezing, coughing, and nasal deformity. Secondary bacterial pneumonia is an important sequel. Grossly, there is atrophy of the scroll-like nasal turbinates. Turbinate atrophy is best evaluated in band-sawed cross-sections of the snout. The definitive diagnosis is by bacterial culture with a specific assay to detect P. multocida cytotoxin.
Actinobacillus pleuropneumoniae and Actinobacillus suis
“APP”, Actinobacillus pleuropneumoniae, is a primary bacterial pathogen: it can cause fatal pneumonia “Contagious pleuropneumonia” without other predisposing causes. Like Mannheimia haemolytica in cattle, it produces toxins that cause lysis of neutrophils and macrophages.
The clinical severity varies: death without premonitory signs; or an acute onset of anorexia, dyspnea, fever, and bloody nasal discharge; or milder signs of coughing and mild respiratory distress.
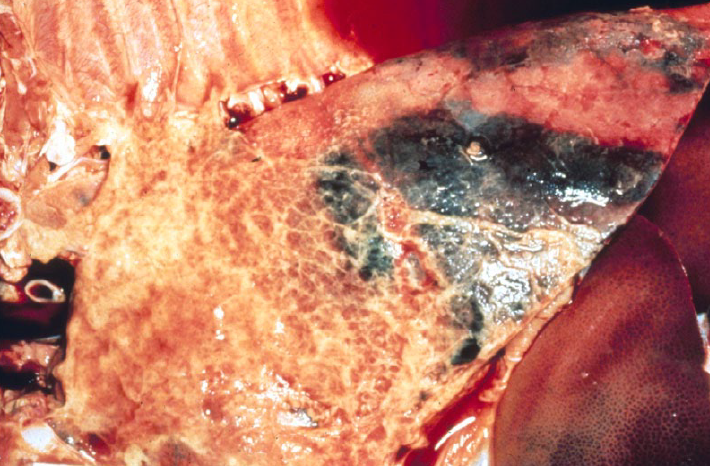
Gross lesions are of severe lobar fibrinous bronchopneumonia with fibrinous pleuritis, usually affecting the caudal lung lobes. Note that this disease is the exception to the rule that the lesions of bronchopneumonia are cranioventral.
Actinobacillus suis causes lung lesions that are identical to APP, or can cause septicemia with multifocal lesions in lung and other tissues.
Other causes of bacterial bronchopneumonia
Pasteurella multocida, Bordetella bronchiseptica, Streptococcus suis, and Glaesserella (Haemophilus) parasuis are opportunistic pathogens that cause bronchopneumonia. The bacterial infection is often secondary to impaired respiratory following infection with Mycoplasma hyopneumoniae, swine influenza, PRRS virus, circovirus, and/or poor ventilation, temperature fluctuations, or stress. This mix of causes is referred to as the “Porcine respiratory disease complex”.
The gross appearance is typical of bronchopneumonia: cranioventral lesions that are firm and red-purple, with or without fibrin on the pleura.
Mycoplasma hyopneumoniae
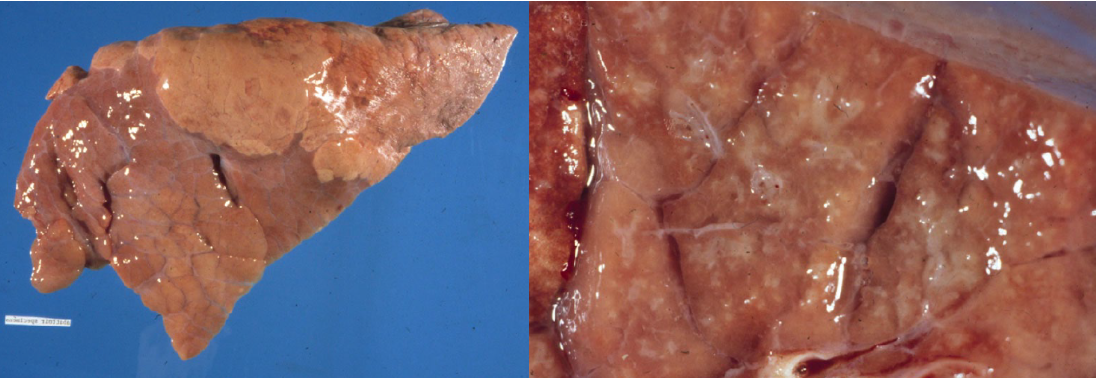
M. hyopneumoniae is a common infection. Most infections are subclinical, or induce a cough that spreads slowly through the herd. Grossly, the lung lesions have a cranioventral distribution, with a “fish flesh” texture that is less firm than a bronchopneumonia, and pink-tan-grey discolouration.
In addition to these direct effects, M. hyopneumoniae infection predisposes to bacterial bronchopneumonia and to PRRS. M. hyopneumoniae adheres to cilia, impairing ciliary function and permitting other bacteria to colonize the lung. M. hyopneumoniae also activates lung macrophages, which helps replication of PRRS virus and worsens this viral disease.
There are many diagnostic tests now available for M. hyopneumoniae:
- PCR from respiratory tract swabs
- Serology on a herd level
- Gross and histologic lesions, confirmed by immunohistochemistry or PCR testing of lung tissue from individual pigs.
- The agent is difficult to isolate in culture, so culture is not a recommended test.
influenza of pigs
Swine influenza virus isolates are classified based on hemagglutinin and neuraminidase proteins. H1N1 is the classical strain, and H3N2 H1N2 are more recent in North America. The strains are only partially cross-protective, so vaccines must be representative of field strains.
The classic clinical appearance is a rapid spread of severe coughing throughout the herd, affecting all age groups, with high morbidity but low mortality. In contrast, some herds instead develop endemic pneumonia with fatalities due to secondary bacterial bronchopneumonia.
Swine-to-human transmission is documented but rare for most strains, with the notable exception of the pandemic H1N1/09 virus that emerged in Mexico in 2009. There is concern that swine may act as “mixing vessels” whereby co-infection of a pig with a highly pathogenic avian influenza virus and a mammalian influenza virus might permit swapping of genetic material that allows the high-virulence avian virus to infect other mammals.
The gross lesions of swine influenza are a cranioventral pattern of rubbery firmness and red discoloration, similar to that seen in cattle with BRSV and dogs with distemper. These lesions are not often observed, because affected pigs do not usually die unless there is secondary bacterial infection. So, the gross lesions are usually of bacterial bronchopneumonia. Histologically, there is necrosis of bronchiolar epithelium with neutrophil infiltration, and there may be proliferation of type II pneumocytes in alveoli.
Definitive diagnosis at necropsy is based on PCR testing, immunohistochemistry, and/or virus isolation on affected lung. Clinical diagnosis is based on demonstrating seroconversion in paired serum samples, or by PCR testing of nasal swabs.
Porcine circovirus
PCV type 2 (PCV-1 is non-pathogenic) emerged in the mid-1990s and was an important cause of disease until highly effective vaccines were developed.
PCV-2 causes PCV2-associated respiratory disease and PCV2-associated systemic disease; the latter was originally known as post-weaning multisystemic wasting syndrome. Most PCV2-infected pigs do not develop disease; immunostimulation or inflammation can activates macrophages and this seems to enhance replication in these cells. Immunostimulation can result from infection with PRRS virus, Mycoplasma hyopneumoniae, influenza virus or porcine parvovirus, so any of these infections can exacerbate PCV-2 infection and lead to PCV2-associated disease. In addition, PCV-2 strains vary in their virulence, and it is this complex mix of virus factors, concurrent infections, and environmental and host factors that determines the impact of infection. PCV-2 infection also infects and impairs the function of pulmonary alveolar and intravascular macrophages, and thus predisposes to other diseases including bacterial pneumonia and septicemia.
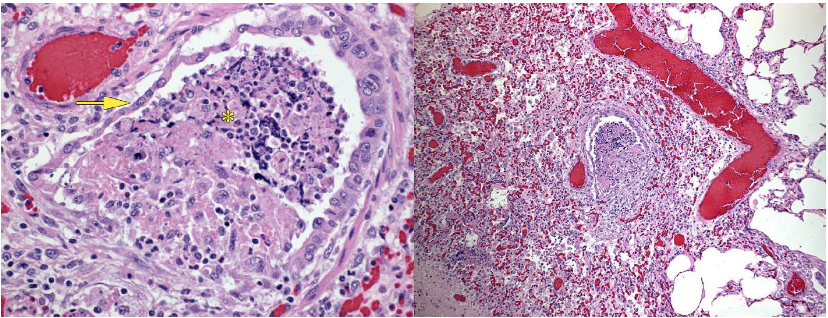
Gross lesions are a diffuse interstitial pneumonia, with firm or rubbery lungs (identical to PRRS) with enlarged lymph nodes, and sometimes enlargement of liver and kidney due to inflammatory cell infiltration. The histologic lesions are depletion of lymphocytes in lymph nodes, ileal lymphoid follicles, and tonsil, with infiltration of macrophages and sometimes the presence of characteristic intracytoplasmic inclusion bodies.
Definitive diagnosis is based on PCR testing or immunohistochemistry of tissues obtained at necropsy, or serology at the herd level.
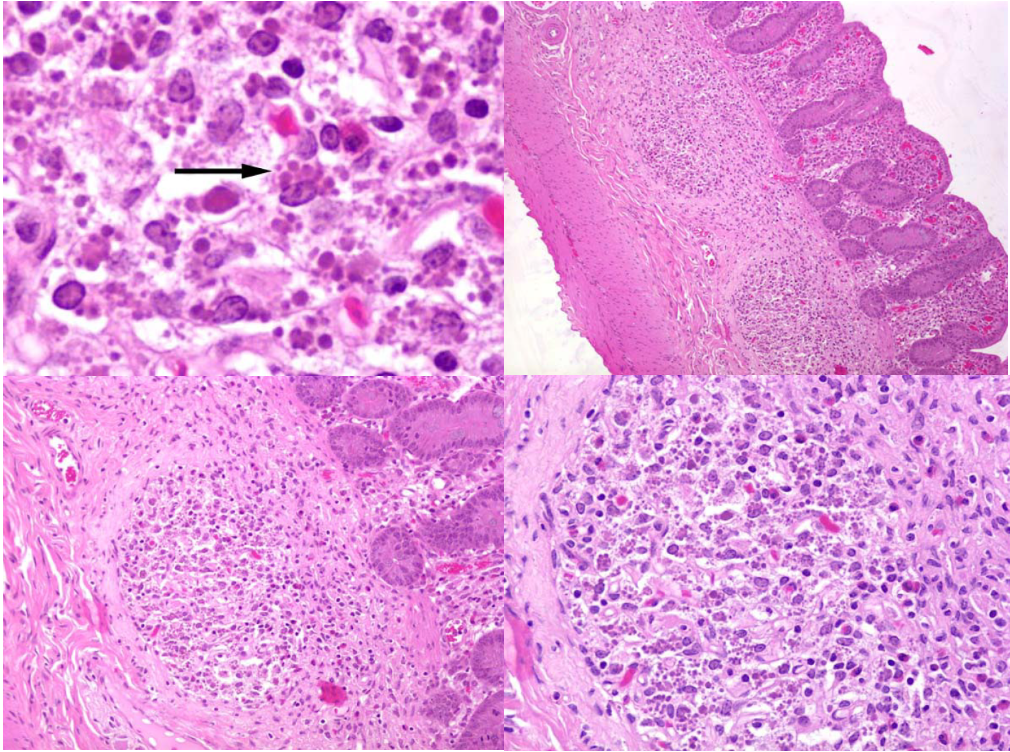
and edematous. Either PCV-2 or PRRS virus could cause this lesion; this pig was infected with both viruses.
Porcine reproductive and respiratory syndrome
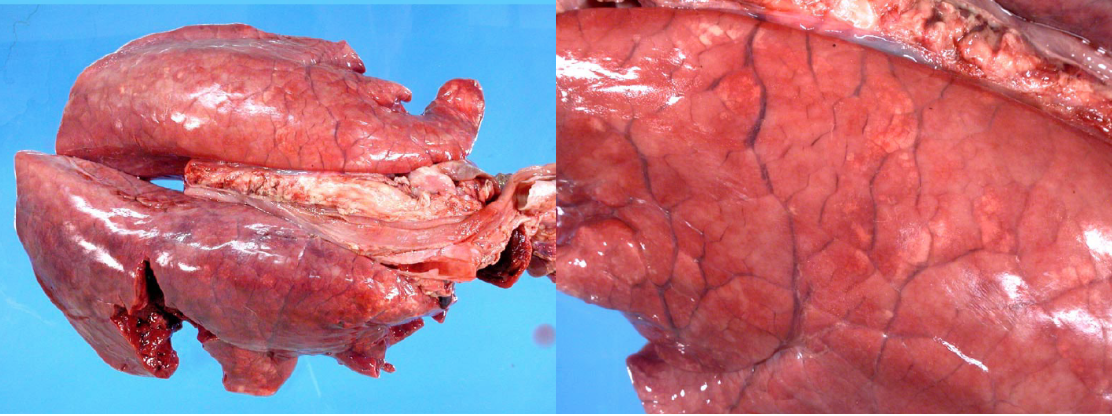
PRRS virus is an arterivirus (RNA virus), first recognized in late 1980s. It is a common infection that causes primary viral pneumonia, but also impairs alveolar macrophage function to allow secondary bacterial pneumonia, and impairs pulmonary intravascular macrophage function to permit septicemia.
The clinical presentation is quite variable due to variation in pathogenicity between viral strains, variation in herd immunity due to exposure and vaccination, and the presence of pigs that shed virus for prolonged periods yet can be clinically normal. Clinically, PRRS manifests as some combination of:
- Dyspnea in suckling or nursery pigs
- Secondary bacterial pneumonia or septicemia in grower-finisher pigs, or less frequently primary viral pneumonia in this age group
- Reproductive failure in sows: late-term abortions, stillbirths, weak-born piglets
Grossly, the lungs are diffusely firm or rubbery, heavy, and fail to collapse (identical to PCV-2 infection). Histologic lesions include thickening of alveolar septa by lymphocytes.
Many tests are available for PRRS, and test selection depends on the clinical circumstances. Gross and histologic lesions can be confirmed as PRRS by immunohistochemistry or PCR testing of lung and tonsil. Herd-level serology detects antibodies in serum, or in oral fluids collected with cotton ropes. Genetic typing of PRRSV isolates (by sequencing the ORF5 gene) can be useful in some herds, to monitor the stability or changes in the virus type over time.
Pneumocystis carinii
This fungal infection is occasionally seen in pigs, but is thought to be secondary to immunosuppression from other diseases.
Question:
What is the key point in the pathogenesis of Pneumocystis infection in foals, pigs, and humans? (Answers are provided at the end of the chapter).
Septicemia and polyserositis
Fibrinous polyserositis in neonatal piglets is caused by E. coli, often due to failure of passive transfer of immunoglobulin.
Septicemia is an important cause of death in weaner pigs, caused by Streptococcus suis, Glaesserella (Haemophilus) parasuis (Glässer’s disease), or Mycoplasma hyorhinis. These infections result in polyserositis, with fibrinous or fibrinopurulent exudates in one or more of the pleural, pericardial, peritoneal cavities, joints, and meninges. Care should be taken to distinguish these exudates from the thin thread-like strands of fibrin that are a normal finding in the abdomen of pigs. Most septicemic pigs have chunks or sheets of fibrin, with abundant fluid.
The diagnosis is based on bacterial culture of swabs taken from the exudates in the above anatomic locations. Septicemia is considered secondary to some other factor, so increased prevalence of septicemia can be an indicator of problems with other infectious agents, ventilation, etc.
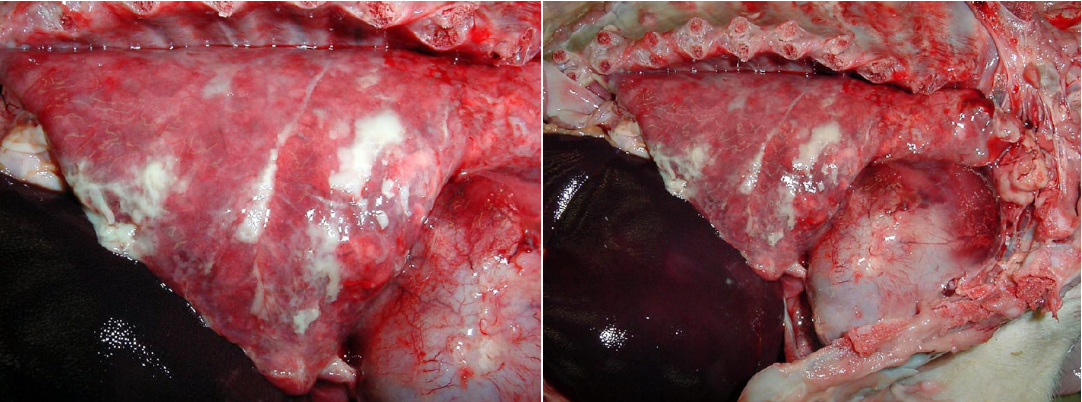
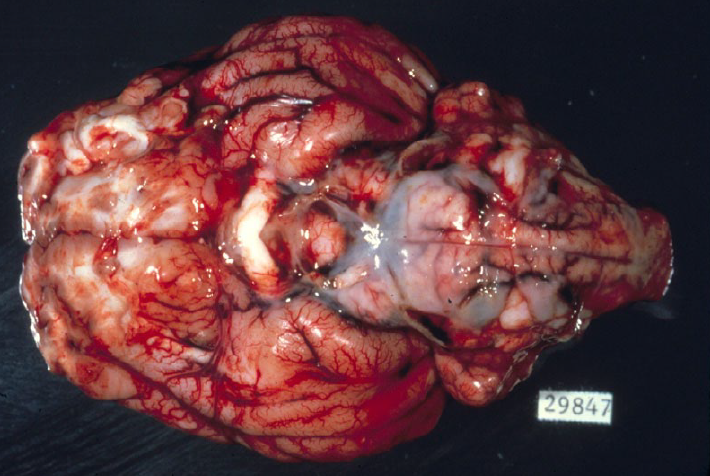
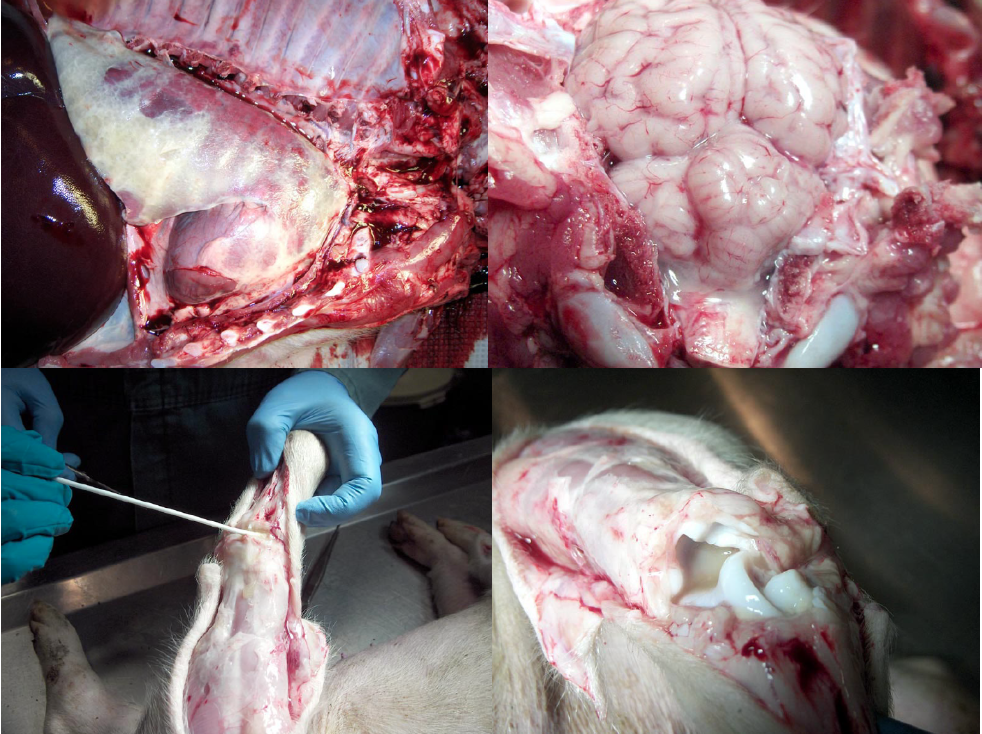
Pseudorabies
Pseudorabies is caused by porcine herpesvirus-1. It is a reportable disease, not present in Canada, but there is risk of introduction from remaining infections in the United States. It mainly causes neurologic disease in baby piglets, but may cause pneumonia in grower-finisher pigs.
Parasitic pneumonia
Metastrongylus spp. lungworms are grossly visible in the bronchi. Earthworms are the intermediate host. Because intensively raised swine are not exposed to earthworms, this disease is now rare, but it probably occurs in backyard pigs.
Migrating larva of Ascaris suum commonly cause liver lesions, and occasionally cause eosinophilic interstitial pneumonia.
Question:
How do Mycoplasma hyopneumoniae and PRRS virus predispose to bacterial pneumonia? (Answers are provided at the end of the chapter).
Question:
What is the cause of fibrinous and necrotizing inflammation of the caudal lung lobe in a grower-finisher pig? (Answers are provided at the end of the chapter).
Question:
List 2 specific causes of diffuse interstitial pneumonia in pigs. (Answers are provided at the end of the chapter).
Question:
What clinical sign is typical with porcine cytomegalovirus infection? (Answers are provided at the end of the chapter).
Question:
What are the five locations of exudates, in pigs with polyserositis? (Answers are provided at the end of the chapter).
Question:
What two bacteria are the most important causes of polyserositis in pigs? (Answers are provided at the end of the chapter).
Question:
Name a reportable disease of pigs that affects the respiratory system. (Answers are provided at the end of the chapter).
Answer:
What is the key point in the pathogenesis of Pneumocystis infections in foals, pigs, and humans?
The fungus Pneumocystis is thought to only cause disease in immunosuppressed individuals.
Answer:
How do Mycoplasma hyopneumoniae and PRRS virus predispose to bacterial pneumonia? (Answers are provided at the end of the chapter).
Mycoplasma hyopneumoniae binds to cilia and prevents mucociliary clearance of inhaled bacteria.
PRRS virus infects macrophages, and suppressed macrophage function accounts for the secondary pneumonia or septicemia.
Answer:
What is the cause of fibrinous and necrotizing inflammation of the caudal lung lobe in a grower-finisher pig? (Answers are provided at the end of the chapter).
Actinobacillus pneuropneumoniae or Actinobacillus suis.
Answer:
List 2 specific causes of diffuse interstitial pneumonia in pigs. (Answers are provided at the end of the chapter).
PRRS virus, Porcine circovirus-2, septicemia, and others.
Answer:
What clinical sign is typical with porcine cytomegalovirus infection? (Answers are provided at the end of the chapter).
Sneezing and nasal discharge in baby pigs. Also stillbirths, mummies, and perhaps “failure-to-thrive” syndrome.
Answer:
What are the five locations of exudates, in pigs with polyserositis? (Answers are provided at the end of the chapter).
Pleura, pericardium, peritoneum, joints, meninges.
Answer:
What two bacteria are the most important causes of polyserositis in pigs? (Answers are provided at the end of the chapter).
Streptococcus suis, Glaesserella (Haemophilus) parasuis, Mycoplasma hyorhinis (Mycoplasma hyopneumoniae causes pneumonia; M. hyorhinis causes septicemia)
Erysipelothrix rhusiopathiae also causes septicemia, but without exudate in body cavities.
Answer:
Name a reportable disease of pigs that affects the respiratory system. (Answers are provided at the end of the chapter).
Pseudorabies.

