3 Patterns and mechanisms of lung disease
morphologic patterns of lung disease
Why classify pneumonia? Making order from chaos: many lung diseases appear clinically and pathologically similar. By classifying disease, we can sort the numerous causes of lung disease into manageable groups that have similar causes, routes of exposure, and disease outcomes. It is then easier to understand the pathogenesis in the case that we are examining, to devise a plan to determine the cause, and to institute appropriate therapy or control measures. It’s helpful to consider 4 patterns of lung disease: bronchopneumonia, airway disease, interstitial lung disease, and embolic pneumonia. We can also include pleural disease among these patterns, and the adventurous might also add pulmonary vascular disease.
- Bronchopneumonia. Inflammatory exudates fill alveoli and bronchioles. This is almost always caused by bacteria that have reached the lung by an airborne route, colonize the air spaces of the alveoli and bronchioles, and elicit an inflammatory reaction that fills these air spaces. Bronchopneumonia typically has a cranioventral distribution of lesions in the lung.
- Airway disease. Inflammation or necrosis specifically affects the bronchi or bronchioles. This may be caused by viruses that infect and kill airway epithelium (e.g. influenza virus), or by inflammatory responses that target airways (e.g. asthma/heaves in horses, or chronic bronchitis in dogs and cats). This pattern has no consistent gross lesions.
- Interstitial lung disease. Damage to the pulmonary interstitium which we can consider mainly as the alveolar septa. Often, the cause damages the alveolar epithelium or endothelium. This may be from viral infection, sepsis, or toxic lung injury. Lesions of interstitial lung disease typically have a generalized diffuse distribution in the lung. Histologic lesions include hyaline membranes, type II pneumocyte proliferation, and interstitial fibrosis.
- Embolic pneumonia. Inflammation resulting from hematogenous infection, usually caused by bacteria or fungi. Embolic pneumonia has a generalized multifocal distribution in the lung.
- Pleural disease. Diseases specifically targeting the pleura are considered in a separate chapter.
- Pulmonary vascular disease. This pattern is useful for specialists, but these diseases are mostly uncommon in domestic animals, and we won’t consider this pattern further in this course.
bronchopneumonia
- Cattle, sheep, and goats: Mannheimia haemolytica, Histophilus somni, Pasteurella multocida, Mycoplasma bovis.
- Swine: Actinobacillus pleuropneumoniae, Pasteurella multocida.
- Horses: Streptococcus zooepidemicus, Rhodococcus equi.
- Dogs: Streptococcus sp., Bordetella bronchiseptica. Aspiration pneumonia is particularly important in dogs, and typically results in mixed growth of E. coli and other bacteria within the lung.
- Cats: Bordetella bronchiseptica, Pasteurella multocida, Streptococcus sp., Mycoplasma felis.
Pathogenesis. Bacteria, entering the lungs via the airways, colonize the air spaces of the bronchioles and alveoli. The inflammatory response chases after the bacteria and therefore targets these air spaces, resulting in exudation of fluid (edema), plasma proteins (fibrinogen/fibrin), and infiltration of neutrophils and macrophages.
Morphology. Histologic appearance. In very early lesions, neutrophils congregate in the lumens of terminal bronchioles and adjacent alveoli. By the time the disease is clinically apparent, alveoli and bronchioles are filled with neutrophils, sometimes fibrin, and edema. But, there is often no damage to the bronchiolar and alveolar epithelium, only filling of the airspaces.
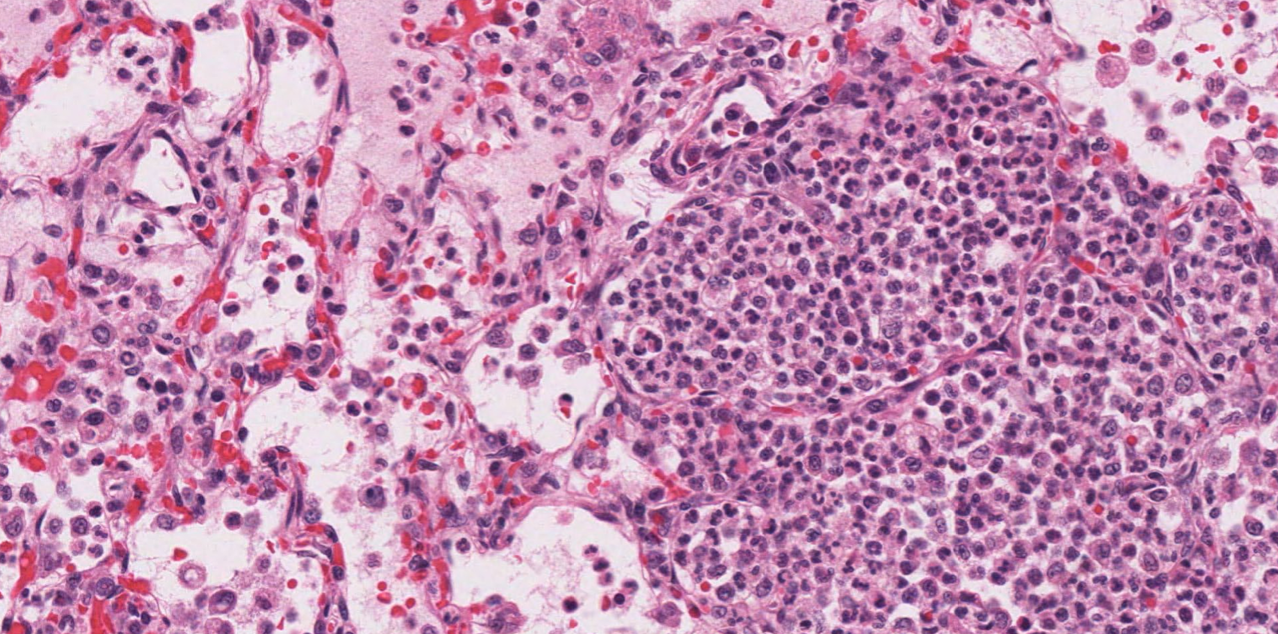
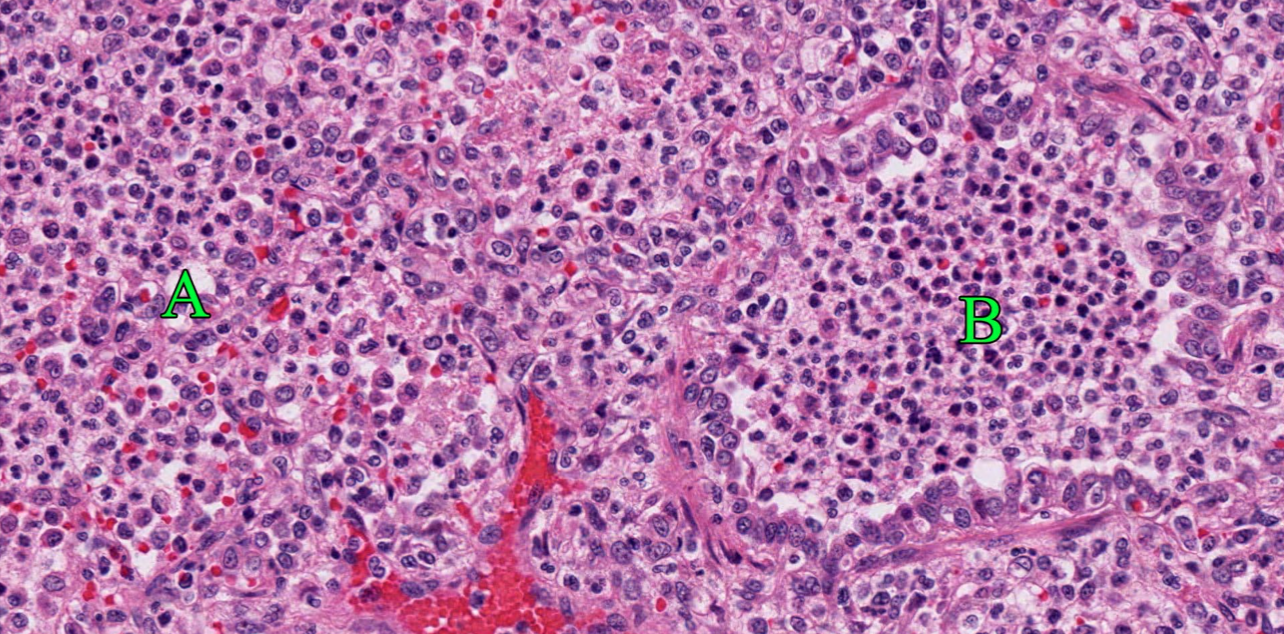
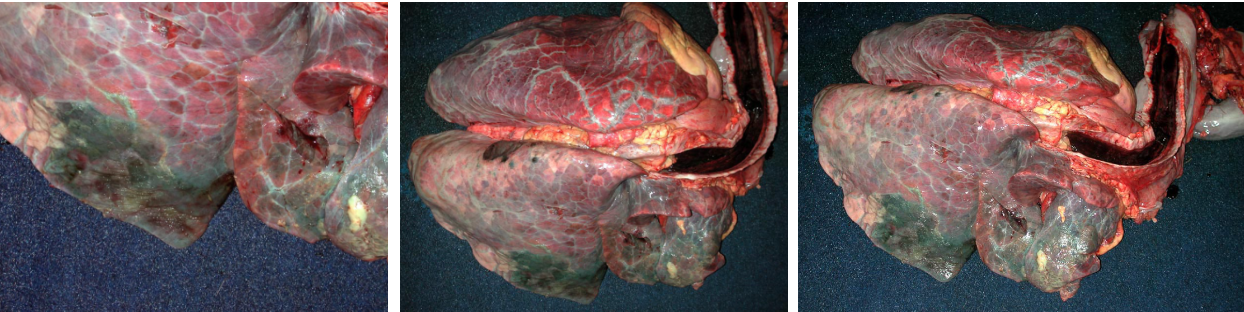
Morphology. Gross appearance. Bronchopneumonia causes a cranioventral distribution of red-purple or tan-white discoloration and consolidation. “Consolidation” means solidification of the lung, with firm texture. This firm texture results from filling of alveoli with exudate (edema, fibrin, neutrophils, macrophages), and the inflammation also causes hyperemia and congestion that imparts the red-purple colour to the lung. As the acute inflammatory response subsides, the hyperemia lessens and leukocyte infiltration increases: this can shift the colour from red to tan/white. Lobular vs. lobar distribution. Severe acute bronchopneumonia develops so quickly that an entire lobe is often affected. This is referred to as a lobar pattern. On the other hand, subacute “smouldering” cases of bronchopneumonia may have a lobular distribution, in which some entire lobules are inflamed while adjacent lobules are unaffected. Recognize that the pathogenesis is the same in both cases, but the acuteness of the lesion and rapidity of spread within the lung is different.
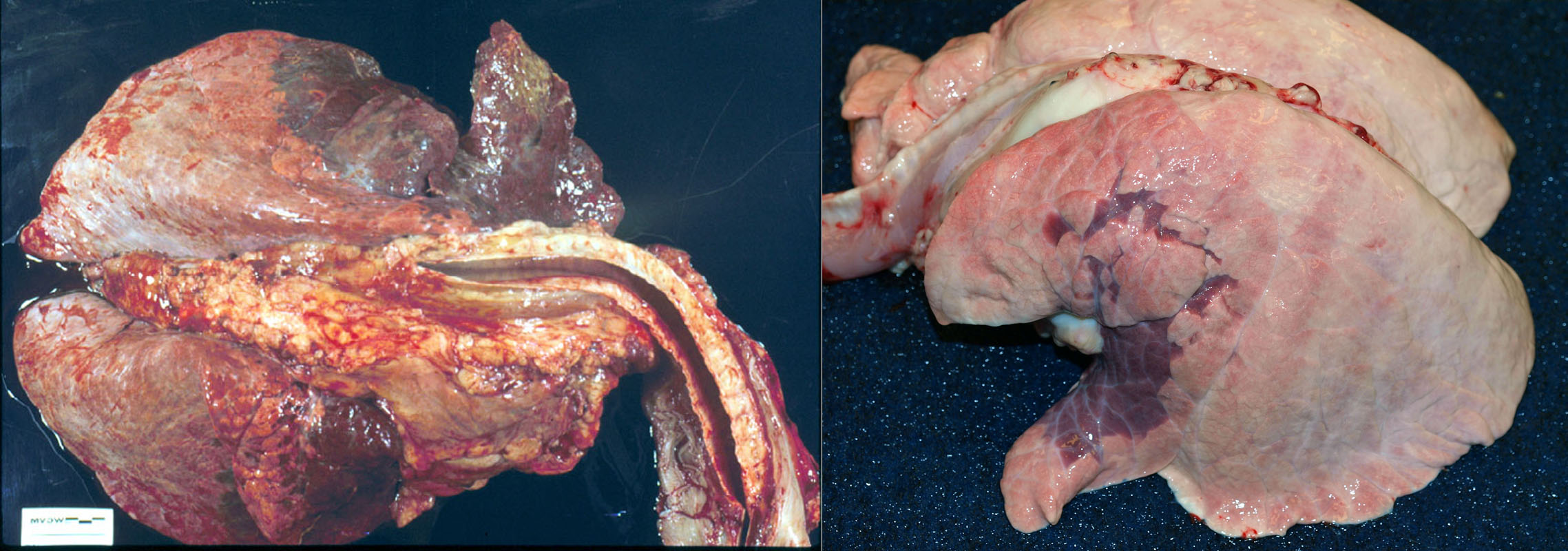
Other lesions that may be present include:
- Fibrin on the pleural surface, as bacteria invade from the alveoli into the pleura. This fibrin will eventually be removed, or organized into fibrous adhesions.
- Fibrin thickening interlobular septa, giving a marbled appearance to the cut section.
- Pus that can be squeezed from the airways, but only in subacute or chronic cases. Don’t confuse the normal foamy content of the airways with pus.
There are two main exceptions to the rule that bronchopneumonia is cranioventral:
- Viral pneumonia may have either a generalized or a cranioventral distribution. BRSV in cattle, influenza in pigs, and distemper in dogs are good examples. When these lesions have a cranioventral distribution, they may be mistaken for bronchopneumonia. However, the lesions tend to be rubbery—not as firm or hard as bronchopneumonia. Nonetheless, you might need histopathology (as well as testing for viruses and bacteria) to accurately distinguish viral from bacterial pneumonia.
- Actinobacillus pleuropneumoniae infection in swine causes bronchopneumonia, but it typically affects the caudal lobes. No one knows why …
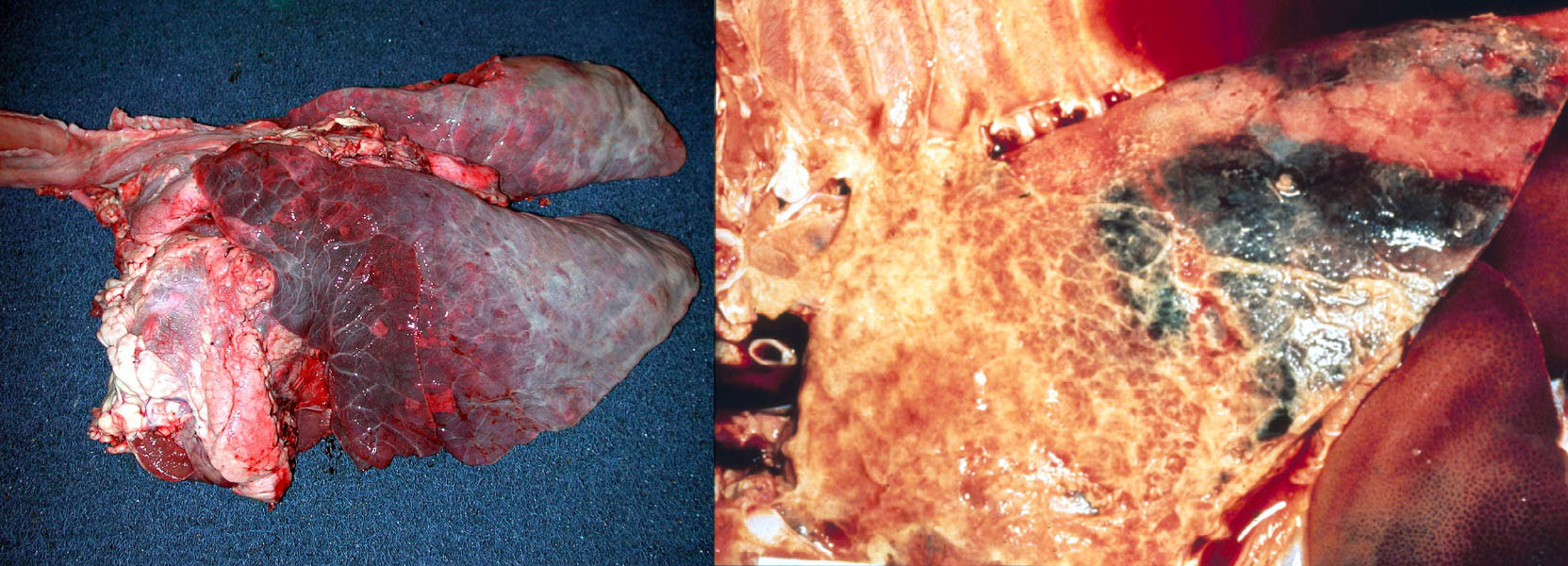
Aspiration pneumonia. Aspiration introduces massive numbers of low-virulence bacteria directly into the lung, overhelming even the healthiest defences.Seen in all species, it is most frequently encountered in dogs and cattle. Important predisposing causes include vomiting (parvoviral enteritis, inflammatory bowel disease), regurgitation (megaesophagus, myasthenia gravis), reduced swallowing reflex (recovery from general anesthesia, laryngeal disease, neurologic disease), cleft palate, or inadvertent passing of a nasogastric tube into the lungs.Recognition of aspiration pneumonia is based on:
- Focal/unilateral distribution, in contrast to the usually bilateral and more-or-less symmetrical distribution of other bronchopneumonias.
- Foul smell, abundant necrosis, and green discoloration; because of the large number of bacteria aspirated and the fact that many are anaerobes.
- Culture reveals a mixed population of environmental bacteria, rather than the 1 or 2 recognized pathogens isolated from other bronchopneumonias.
- Plant material (herbivores) or meat (carnivores) may be visible histologically, with an associated inflammatory reaction (eg. neutrophils around the plant material).
In carnivores (especially dogs), the lesions are as described above if the aspirated material contains food or numerous bacteria. But alternatively, dogs can aspirate relatively sterile gastric acid that damages bronchiolar and alveolar epithelium, and thus makes an interstitial lung disease. This is uncommon, and most dogs with aspiration pneumonia have cranioventral, focal, lobar, or unilateral pneumonia as described above.
Question:
In a dog with aspiration pneumonia, what predisposing lesions might you observe elsewhere in the body? (Answers are provided at the end of the chapter).
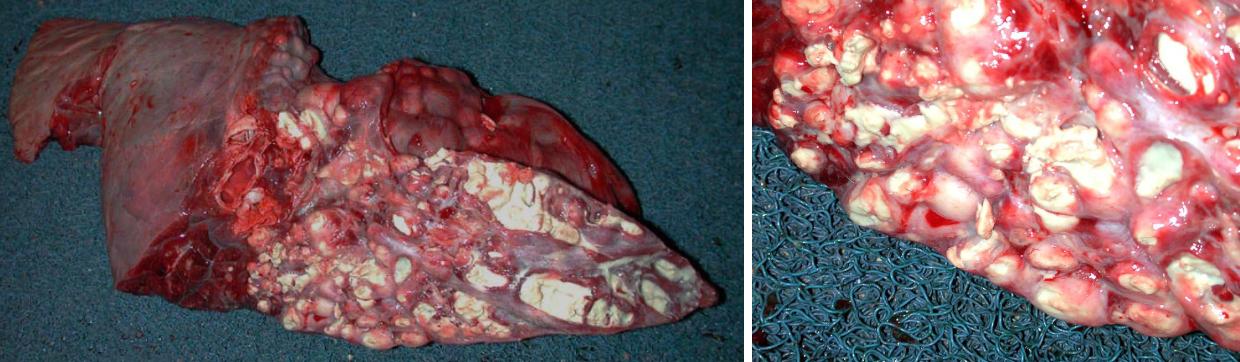
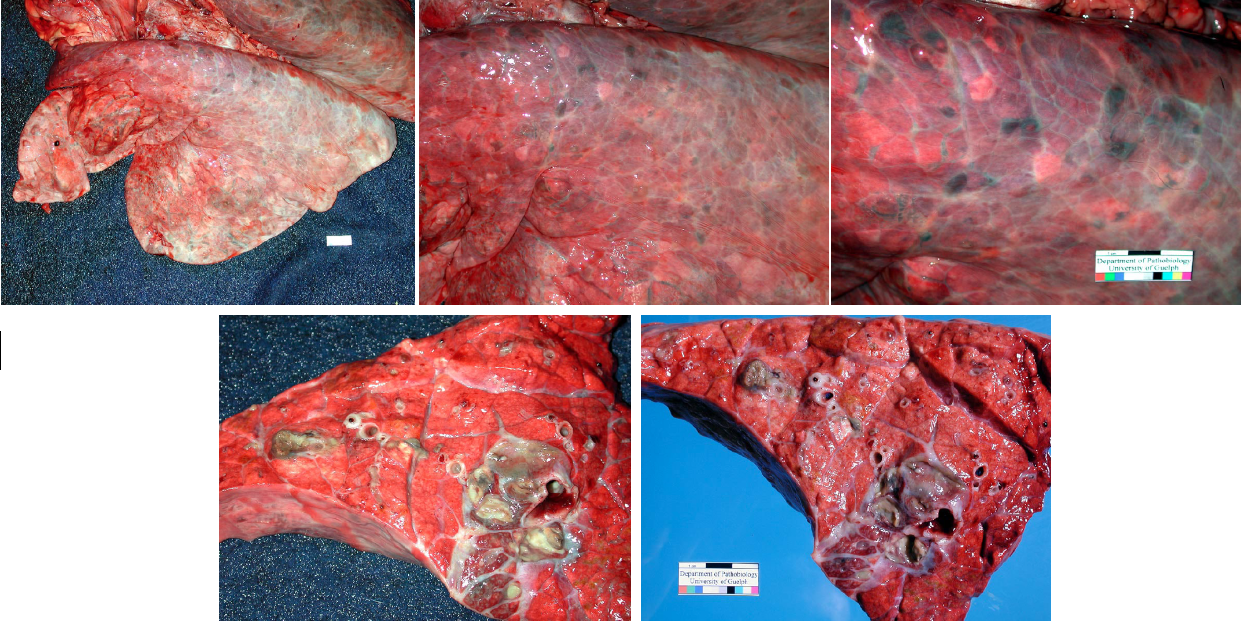
Sequelae. The lesions of bronchopneumonia can resolve if the infection is eliminated. Fibrin is removed by plasmin (fibrinolysis), and neutrophils undergo apoptosis. If the infection cannot be eliminated, then the inflammatory response will continue, and sequelae include chronic suppurative bronchopneumonia, abscess formation, bronchiectasis, and sequestrum formation.
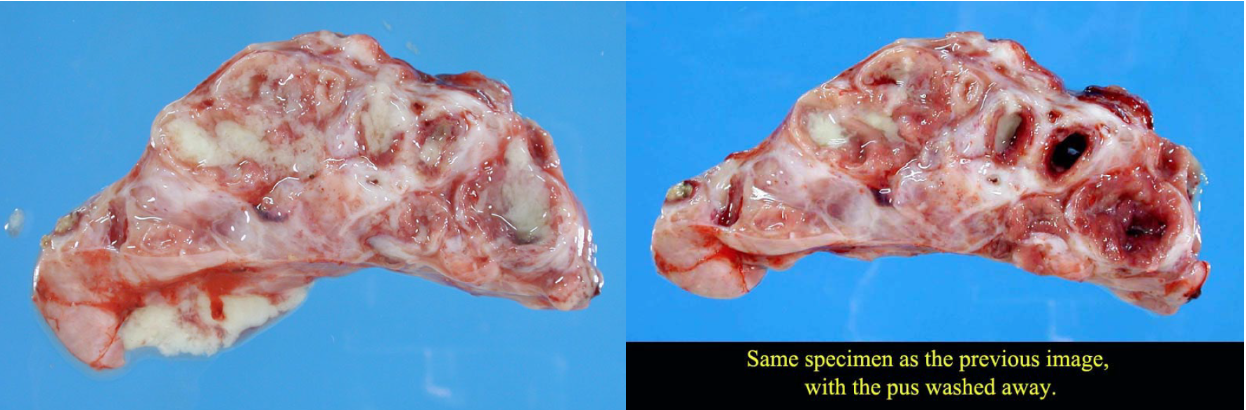
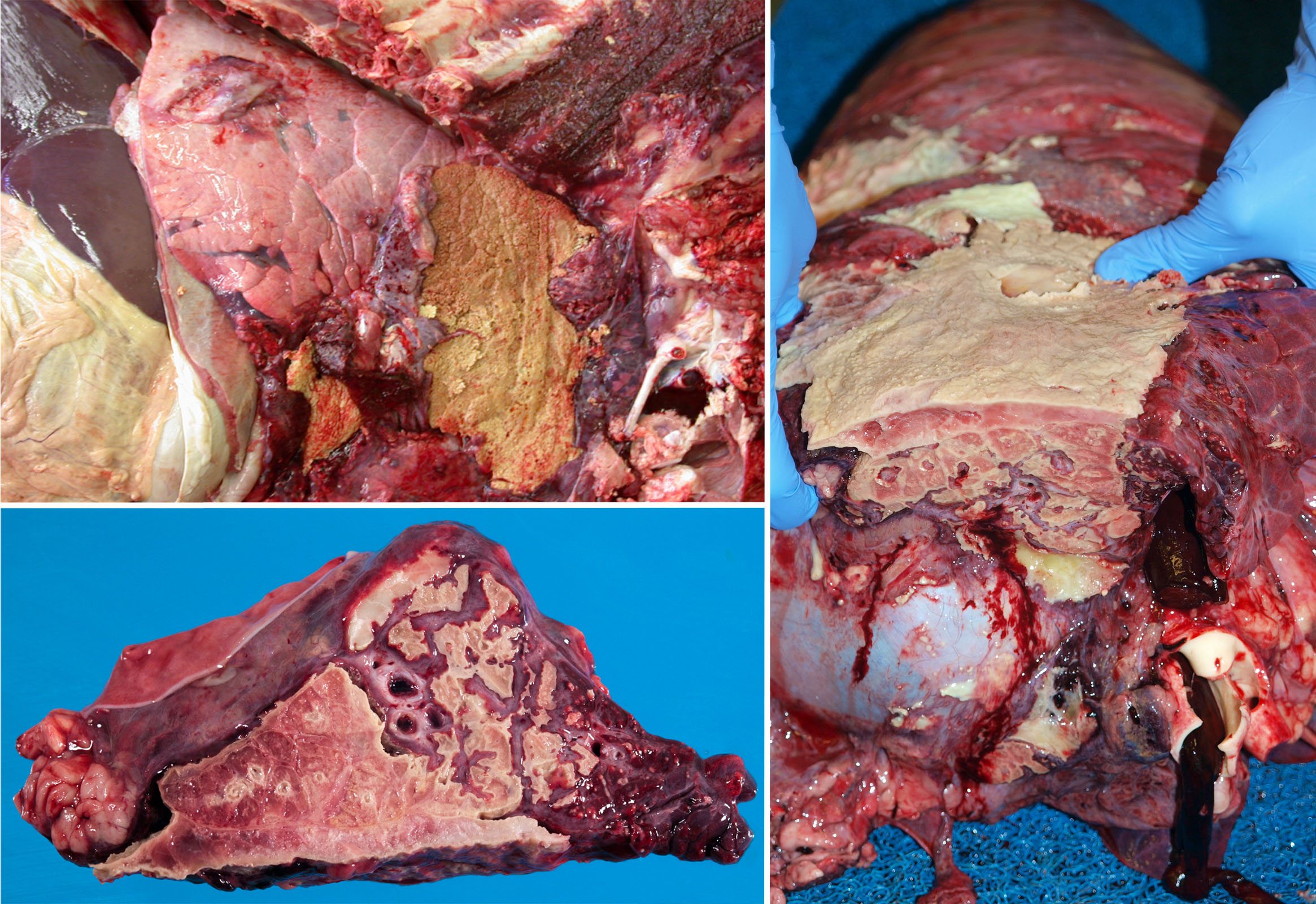
- Abscesses form when the bacterial infection can be contained but not eliminated. The typical appearance is cranioventral consolidation with multifocal pus-filled encapsulated nodules. Since antibiotics cannot penetrate well, abscesses (as well as bronchiectasis and sequestra) can persist for the life of the animal, and clinical signs often relapse after antibiotic therapy is discontinued. Animals with lung abscesses may be clinically normal or have chronic respiratory signs. In other cases, erosion into a bronchus or a blood vessel can cause fatal bronchopneumonia, sepsis, or pulmonary hemorrhage with epistaxis.
- Bronchiectasis: greatly dilated bronchi that are filled with pus. This may appear similar to abscesses, but forms cylindrical structures that eventually join with the larger bronchi. Bronchiectasis develops as a result of chronic bacterial infection in which the ensuing inflammatory exudates obstruct airways and cannot be expelled. The leukocytes’ proteolytic enzymes and oxygen radicals damage the bronchial wall, and the bronchus dilates because the wall is weakened. Like abscesses, bronchiectasis is always chronic.
- Sequestrum formation: a large mass of necrotic lung tissue. Because macrophages cannot penetrate far into the dead avascular tissue, it cannot be readily removed.
Chronic sequelae of bronchopneumonia: abscesses, bronchiectasis, and sequestra.
Question:
In a puppy with parvoviral enteritis, how would you distinguish whether bacterial infection of the lung was a consequence of vomiting or of reduced barrier function of the intestinal mucosa? (Answers are provided at the end of the chapter).
Question:
Necropsy of a dog reveals consolidation and reddening of the right middle lung lobe, reddening but normal texture of the remainder of the lung, and isolation of Enterobacter sp., E. coli , and Citrobacter sp. Indicate the expected pathogenesis of the disease in this dog. (Answers are provided at the end of the chapter).
Question:
What bacterial pathogen is an important primary cause of bronchopneumonia in both dogs and cats? (Answers are provided at the end of the chapter).
Question:
What are the three most important bacteria causing bronchopneumonia in cattle? (Answers are provided at the end of the chapter).
Question:
Let’s review the morphologic patterns of lung disease. Use the clues to identify the pattern for each column, then complete the missing information. (Answers are provided at the end of the chapter).
| Name of this pattern | Pattern #1 ??? | Pattern #2 ??? | Pattern #3 ??? | Pattern #4 ??? |
| Cause | Bacteria | Viruses, bacteria, hypersensitivity | ||
| Route of exposure | Airway | Usually airway | ||
| Gross distribution of lesions | Generalized multifocal | |||
| Key histologic feature | Hyaline membranes, type II pneumocyte proliferation, interstitial fibrosis |
airway disease
Also known as: bronchitis/bronchiolitis/bronchiolar necrosis
Causes of airway disease include infectious agents, hypersensitivity reactions, and toxins. In this discussion of lung disease, the “airways” refers to the bronchi and bronchioles; trachea and nasal tissues may also be affected.
Viruses infect airway epithelial cells and induce acute epithelial injury. Some of these viruses also infect and damage alveolar epithelium. Some viral respiratory infections are:
- Cattle: Bovine respiratory syncytial virus, bovine herpesvirus-1 (infectious bovine rhinotracheitis), bovine coronavirus, bovine parainfluenza virus.
- Sheep and goats: BRSV. (Maedi-visna virus and enzootic nasal tumour virus are important but don’t cause airway disease).
- Swine: Influenza (PRRS virus and Porcine circovirus are important but they cause interstitial lung disease rather than airway disease).
- Horses: Equine herpesviruses-1 & -4, equine influenza virus.
- Dogs: Canine coronavirus, canine distemper virus, canine adenovirus-2, canine parainfluenza virus, canine influenza virus.
- Cats: Feline herpesvirus, feline calicivirus.
Bacterial infections can cause bronchitis, although severe infections more typically result in bronchopneumonia.
Hypersensitivity reactions are important causes of airway disease. Examples include asthma (heaves) in horses, asthma in cats, and chronic bronchitis in dogs and cats.
Toxins & chemical irritants can cause airway disease, alveolar disease, or both. Smoking, dusts, & gases directly irritate or kill the airway epithelium, leading to bronchitis and bronchiolitis. Other toxins, such as 3-methylindole, must be metabolized to reactive intermediates. Clara cells contain cytochrome p450 mixed-function oxidases (similar to periacinar hepatocytes) and transform xenobiotics to reactive intermediates in the process of detoxifying them. However, these reactive intermediates may induce tissue damage before they are conjugated and excreted.
Morphology of airway disease. There are often no gross lesions. Histologic lesions include inflammation centred on airways, and/or necrosis of ciliated epithelium.
Functional consequences of airway disease. These diseases cause airway narrowing mainly as a result of smooth muscle contraction (bronchoconstriction), triggered by inflammatory mediators such as leukotrienes, other eicosanoids, or cytokines. Other contributors to airway obstruction are inflammation and edema of the airway wall, and exudates and necrotic cells within the lumen. The functional effects of airway obstruction are:
- Increased resistance to airflow through the airways
- Increased respiratory effort (dyspnea)
- Failure to ventilate the alveoli, thus leading to hypoxemia and hypercapnia.
Recall the physiologic reflex by which alveolar hypoxia (as a result of airway obstruction) triggers vasoconstriction. In cases of mild bronchopneumonia, this is beneficial because it minimizes perfusion of the non-ventilated lung and prevents shunting of non-oxygenated blood past the non-ventilated area of lung (ventilation-perfusion matching). However, if alveolar hypoxia is widespread (due to widespread airway lesions), then generalized pulmonary vasoconstriction may lead to pulmonary hypertension and cor pulmonale. Cor pulmonale refers to pulmonary hypertension and right heart failure as a consequence of lung disease.
Sequelae and resolution
- Optimal sequence of events. The airway epithelium heals quickly following an acute one-time insult, as follows: Viral infection → Necrosis → Sloughing of necrotic epithelial cells with exposure of basement membrane → Flattening and stretching of surviving epithelial cells to cover the basement membrane → Clearing of exudates by mucociliary clearance, macrophages and proteolytic enzymes → Proliferation of surviving epithelial cells → Differentiation into normal epithelium. Healing is usually perfect following viral infections, but may not occur if there is repetitive injury (e.g. smoking in humans, irritant gases, chronic infections), or if there is overwhelming damage to the epithelium.
- Increased susceptibility to bacterial pneumonia. Necrosis of the ciliated airway epithelium impairs mucociliary clearance, thus predisposing to opportunistic bacterial infection of the lung.
- Atelectasis and/or emphysema may occur as a result of airway obstruction.
- Bronchiectasis, described above in the section on bronchopneumonia, can occur due to chronic bronchitis.
- Bronchiolitis obliterans fibrosa (aka obliterative bronchiolitis, intrabronchial polyps). If exudate in bronchioles cannot be removed, it is organized like any wound: fibroblasts infiltrate and synthesize collagen production, and there is neovascularization and re-epithelialization. However, because this occurs in the airway lumen, it leads to airway obstruction by an epithelium-covered mass of fibrous tissue in the airway lumen. This fibrous tissue obliterates the bronchiolar lumen, leading to alveolar hypoxia, and this fibrotic area of lung is permanently non-functional. The clinical significance depends on how much of the lung is so affected.
- Mucous and squamous metaplasia, neoplastic transformation. Ongoing damage may cause the bronchial epithelium to adapt to the harsh conditions. Although this sequence of events is important in smoking-related diseases in humans (chronic obstructive pulmonary disease and pulmonary carcinoma), it is uncommonly recognized as an important sequel in animals. Chronic airway injury may manifest as mucous hyperplasia (increased number of mucus-secreting goblet cells in the airway epithelium) or squamous metaplasia (replacement of the ciliated epithelium with squamous epithelium). These are adaptive responses: the mucus blankets the harassed epithelium and protects it from further injury, and the squamous epithelium is more resistant to damage than ciliated epithelium. But metaplasia has a dark side. There are no more cilia, so mucociliary clearance is impaired, and this predisposes to bacterial pneumonia. And over time, the epithelial cells experiencing repeated bouts of DNA damage, oxidative injury, and attempts to repair are prone to undergo neoplastic transformation.
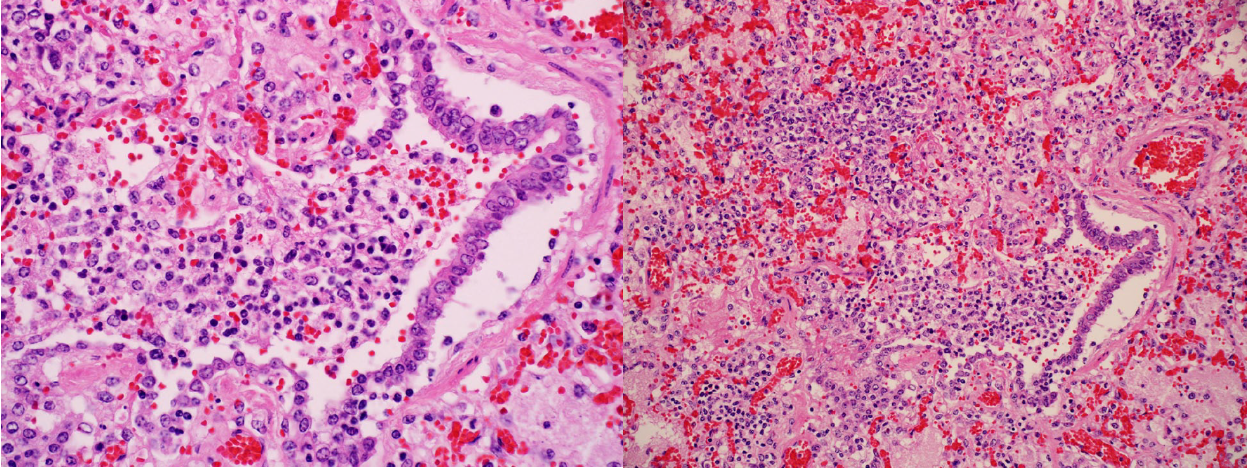
Question:
List the two most important viral respiratory infections of cattle, and two of cats. (Answers are provided at the end of the chapter).
Question:
List three mechanisms of airway obstruction in a pig with influenza. (Answers are provided at the end of the chapter).
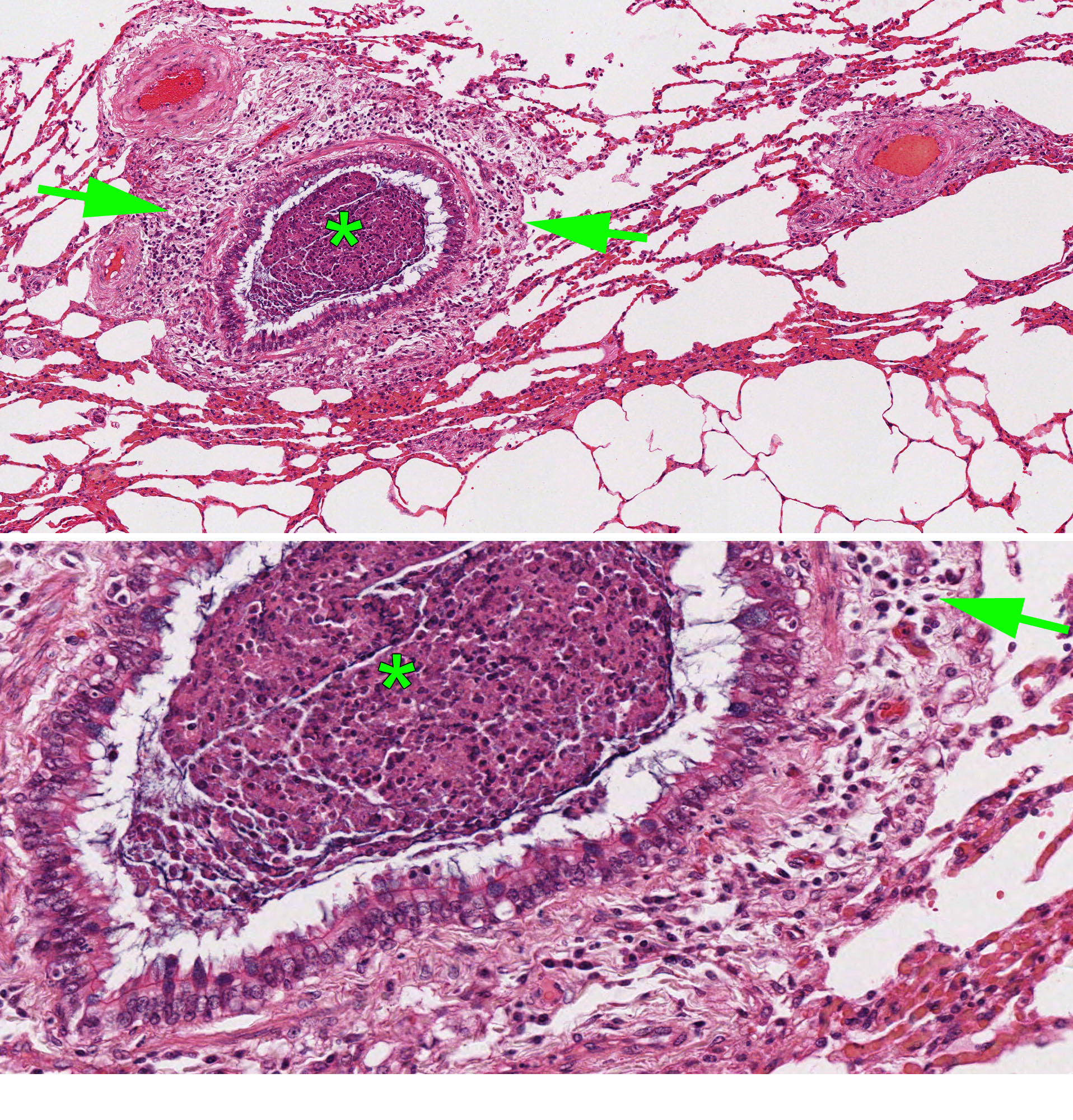
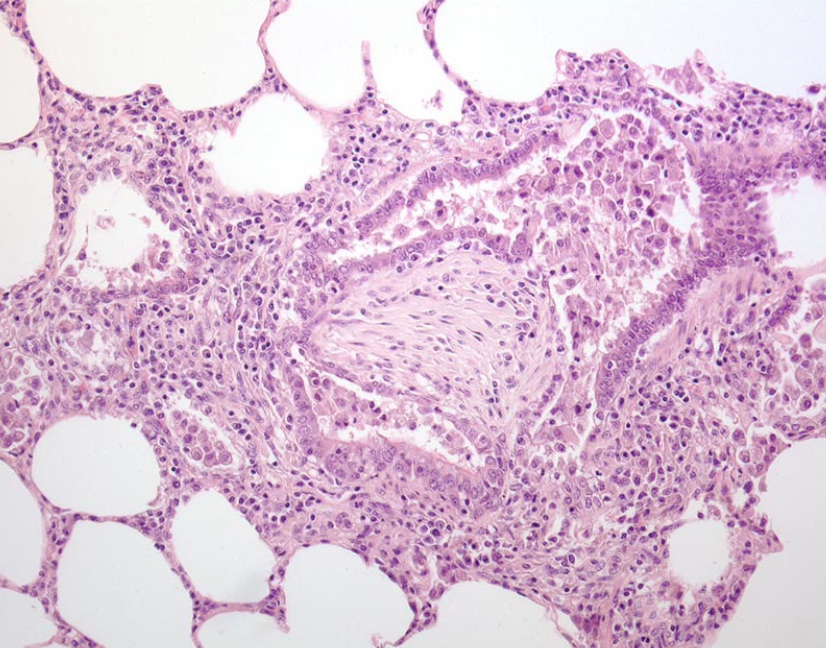
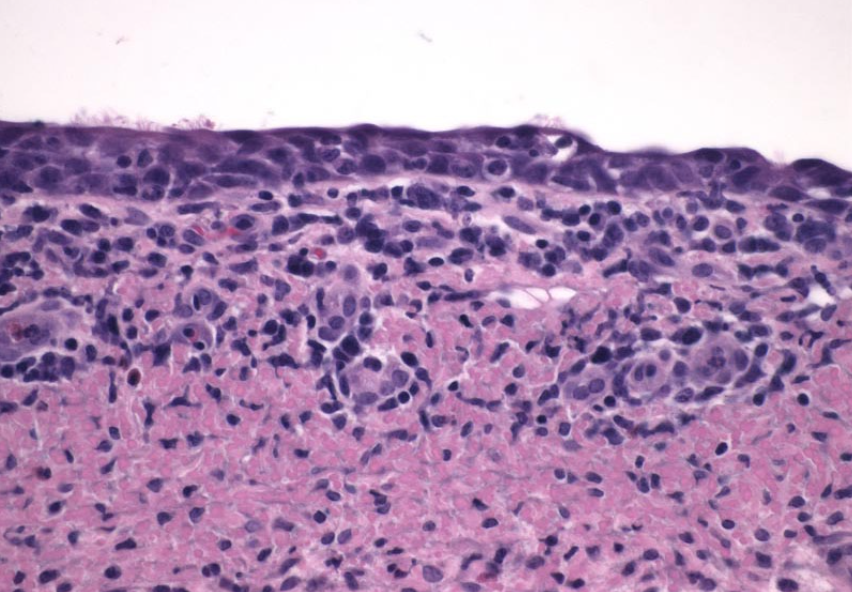
Question:
What is the difference between bronchiectasis and bronchiolitis obliterans, with respect to morphology and cause? (Answers are provided at the end of the chapter).
interstitial lung disease
also known as “interstitial pneumonia”
Make some tea and get a snack, interstitial lung disease is the hardest of these patterns to comprehend.
Causes of injury
- Infectious agents: many viruses, migrating larva (e.g. Dictyocaulus, Ascaris suum), protozoa (e.g. Toxoplasma)
- Sepsis
- Toxins that are activated by biotransformation: 3-methylindole (fog fever), paraquat, mouldy sweet potatoes, Perilla mint, … These are rarely recognized in Ontario.
- Toxins that cause direct injury without biotransformation: chlorine gas, 100% oxygen, acid aspirated from vomitus, silo gas, noxious gases
- Chemical injury from aspiration of gastric acid, or smoke inhalation from fires, or inhalation of toxic gases like ammonia, chlorine, nitrogen oxides (silo gas)
- Hypersensitivity: allergic alveolitis in housed adult dairy cattle. This is uncommon.
- Pneumoconiosis: inhalation of inorganic dusts can cause chronic interstitial lung disease. Asbestosis, silicosis, and many other occupational exposures are well-recognized in humans, but are rare in domestic animals.
Question:
Give one example of a pneumoconiosis. (Answers are provided at the end of the chapter).
Pathogenesis. The above causes of interstitial lung disease share the common feature of causing damage to alveolar epithelium or endothelium. Viruses and protozoa infect and cause necrosis of alveolar epithelial cells. Chlorine gas directly damages the alveolar epithelium, while 3-methylindole is metabolized by type II pneumocytes to reactive intermediates that injure the epithelium. Sepsis causes a “cytokine storm”, in which high levels of circulating cytokines increase vascular permeability in the lung.
The endothelium and the epithelium are both important barriers to water movement across the air-blood barrier. Damage to either of these cell types causes alveolar edema.
In the acute stages of interstitial lung disease, hyaline membranes form in the alveoli. Hyaline membranes are a mixture of plasma proteins, surfactant proteins, and dead type I pneumocytes. When the alveolar epithelium is damaged, these substances form a membrane that lines the inner surface of the alveolus. Think of hyaline membranes as a scab covering the alveolar surface. Hyaline membranes are a diagnostic indicator that there has been damage to the alveolar epithelium. The epithelium itself is too thin to see, but the hyaline membranes tell us it has been injured.
Type II pneumocytes are the regenerative cell in the alveolus. Starting at 3 days after injury to type I pneumocytes, the type II cells proliferate. At this stage, the alveolus becomes lined by cuboidal type II pneumocytes. Although this is a necessary step in repair of alveolar injury, lung function is still abnormal because the thick type II pneumocytes are a barrier to oxygen exchange between the blood and the air.
Following an acute one-time injury, type II pneumocytes eventually differentiate into type I pneumocytes, and lung function returns to normal. In less favorable circumstances, type II pneumocytes persist and interstitial fibrosis may develop. This permanently impairs gas exchange, and reduces the elasticity or compliance of the lung. The balance between epithelial repair (optimal) and repair by fibrosis (a permanent barrier to gas exchange) depends on persistence of the stimulus and extent of injury (and perhaps also the adequacy of the repair process). Chronic persistent stimuli (such as ongoing exposure to irritant chemicals, persistent virus infections, dusts particles, or hypersensitivity) are more likely to cause fibrosis than one-time insults (such as viral infection). Similarly, chronic inflammatory responses may heal by fibrosis. Finally, with severe damage, fibrosis may develop before the tissue has time to heal.
Functional effects. The obvious effect of alveolar edema, hyaline membranes, type II pneumocytes, and interstitial fibrosis is to act as an obstruction to gas exchange in the alveolus. Less obvious is the reduction in the elasticity or compliance of the lung: it takes more work to inflate a fibrotic lung. Finally, because the lung doesn’t expand as much during inspiration, nor collapse as much during exhalation, the functional lung volume is reduced. So, interstitial lung disease causes hypoxemia, reduced lung compliance, increased work of breathing, and reduced functional lung volume.
Morphology. Lesions of interstitial lung disease are throughout the lung (rather than localized of affecting a single lobe). They are usually in a diffuse distribution, or less often in a lobular or “checkerboard” distribution. These lungs fail to collapse and sometimes have impressions of the ribs on their pleural surface. They are firmer than normal (but this is less obvious than for bronchopneumonia), heavy and wet because of edema, and often reddened. Interlobular edema and/or interlobular emphysema may be present in cattle, pigs and horses.
Histologic features include edema and hyaline membranes in acute stages, or proliferation of type II pneumocytes and interstitial fibrosis if chronic.
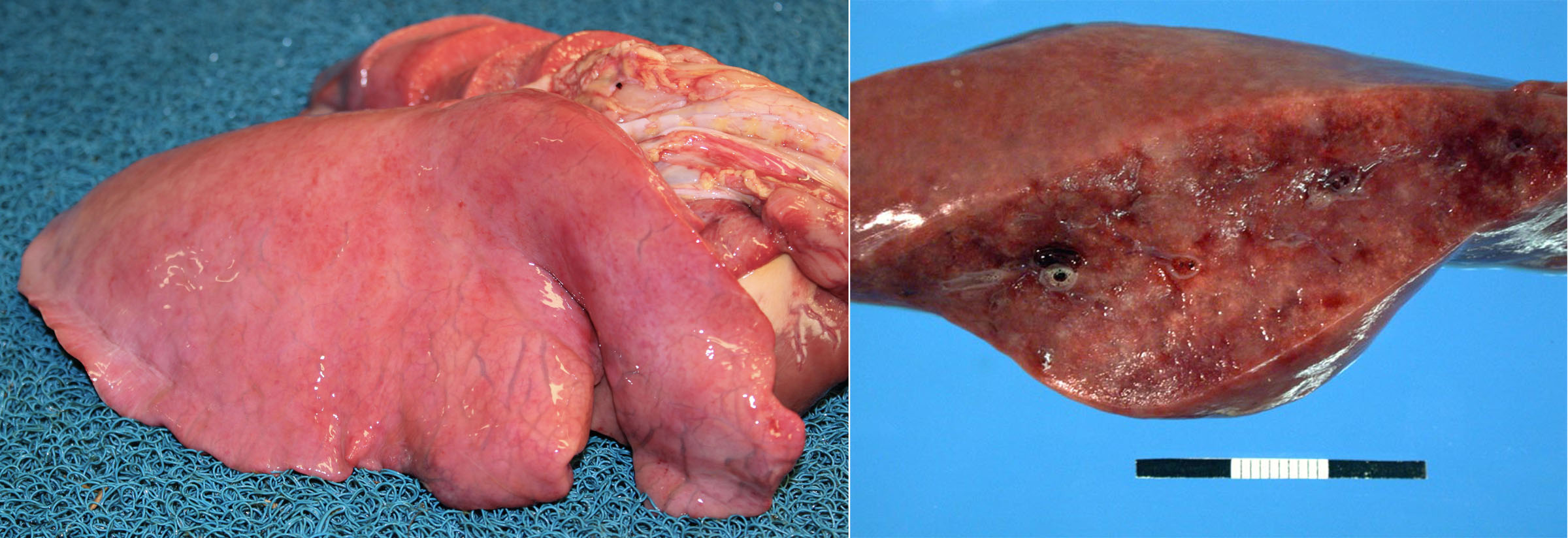
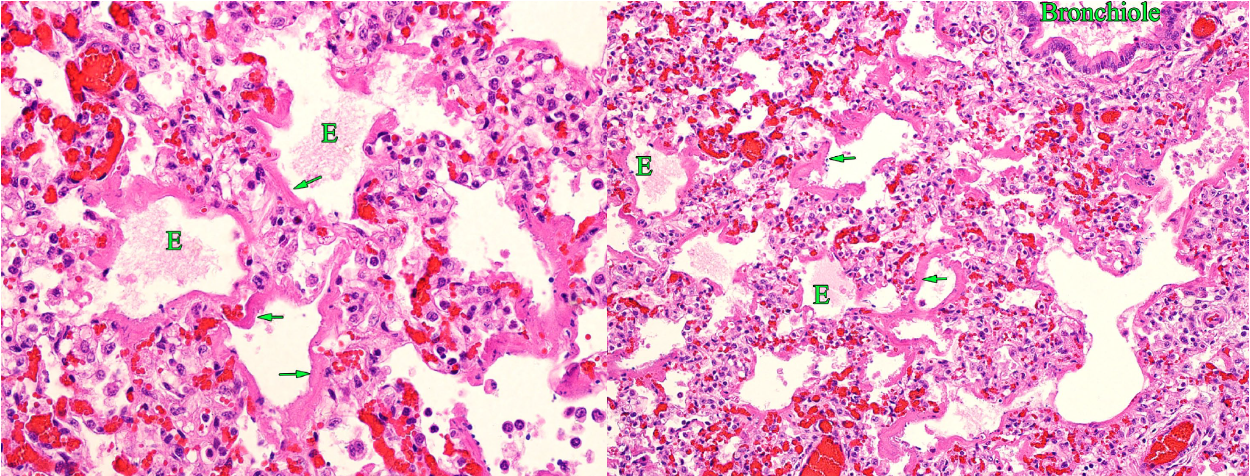
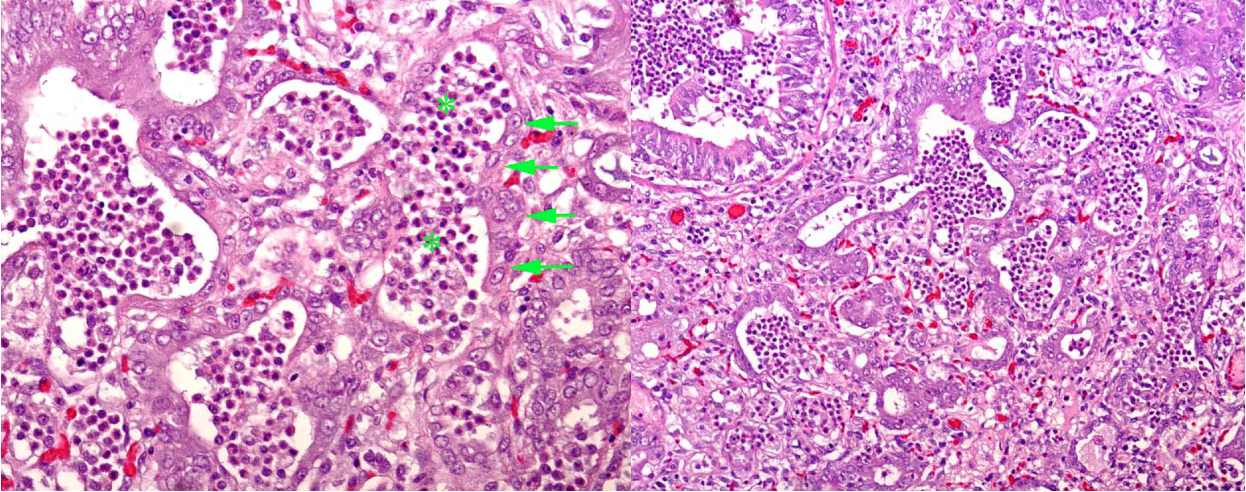
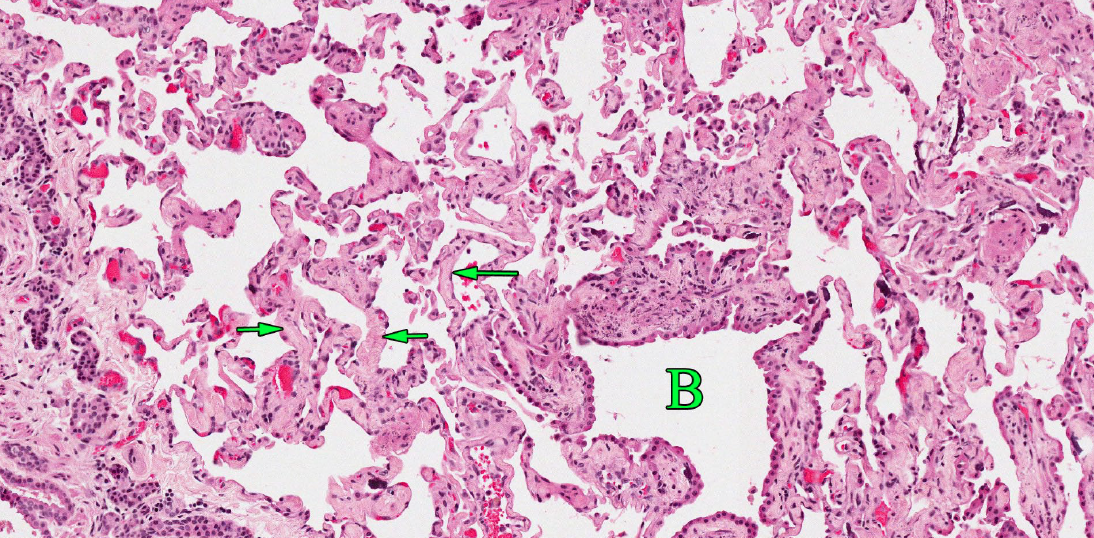
Question:
What are the two main features of chronic interstitial lung disease? (Answers are provided at the end of the chapter).
Sometimes, interstitial lung disease and bronchopneumonia occur concurrently, and these cases can be tricky to evaluate grossly. The obvious lesion in such cases is cranioventral bronchopneumonia, but the more normal caudodorsal lung is also diffusely firmer than normal and usually fails to collapse. These cases may have a chronic history of relatively mild respiratory disease (caused by the bronchopneumonia) with acute severe fatal exacerbation (caused by the interstitial lung disease). This pattern is recognized, for example, in foals with relatively mild bronchopneumonia caused by Rhodococcus, or in feedlot cattle with chronic bronchopneumonia caused by Mycoplasma bovis and acute interstitial pneumonia of uncertain cause.
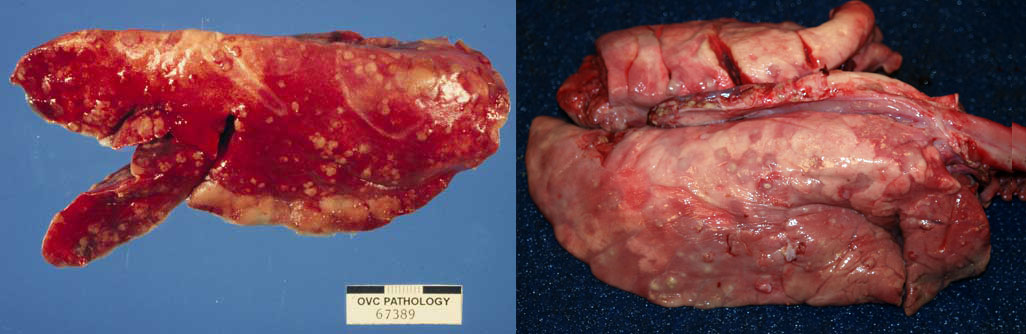
Embolic pneumonia
This form of pneumonia results from blood-borne bacteria or fungi. If an abscess ruptured into a large vein, then massive numbers of bacteria would suddenly attack the lung, and this would cause a diffuse interstitial lung disease as described above. But here we are discussing a less fulminant disease, where low numbers of bacteria might seed a tiny area of the lung today, and another cluster colonizes a different area of lung tomorrow, and another the next day. Each of these tiny colonies grows to form an abscess, and those abscesses together have the generalized multifocal distribution that indicates embolic pneumonia. Embolic pneumonia often originates from right-sided valvular endocarditis, omphalitis, jugular venipuncture, traumatized and infected ears in pigs, liver abscesses in feedlot cattle, or udder cleft dermatitis in dairy cows. The differential diagnoses are metastatic neoplasia, fungal infections such as blastomycosis, or inhalation of foreign material such as wood shavings.
Answer:
In a dog with aspiration pneumonia, what predisposing lesions might you observe elsewhere in the body?
Lesions in other organs that predispose to aspiration would include megaesophagus, persistent right aortic arch, cleft palate, thymoma (causing myasthenia gravis), enteritis (parvovirus, inflammatory bowel disease, brain lesions, or a surgical incision associated with recent anesthesia.
Answer:
In a puppy with parvoviral enteritis, how would you distinguish whether bacterial infection of the lung was a consequence of vomiting or of reduced barrier function of the intestinal mucosa?
Vomiting would induce aspiration pneumonia, which would usually cause a localized cranioventral lesion of bronchopneumonia. Reduced mucosal barrier function would lead to sepsis, and the resulting lung lesions would be diffuse throughout the lung (interstitial pattern).
Answer:
Necropsy of a dog reveals consolidation and reddening of the right middle lung lobe, reddening but normal texture of the remainder of the lung, and isolation of Enterobacter sp., E. coli , and Citrobacter sp. Indicate the expected pathogenesis of the disease in this dog.
The presence of a localized lung lesion and a mixed culture of enteric bacteria suggests aspiration pneumonia.
Answer:
What bacterial pathogen is an important primary cause of bronchopneumonia in both dogs and cats?
Bordetella bronchiseptica.
Answer:
What are the three most important bacteria causing bronchopneumonia in cattle?
Mannheimia haemolytica (and the related pathogen Bibersteinia trehalosi)
Histophilus somni
Pasteurella multocida
Mycoplasma bovis
Answer:
Reviewing the introduction to morphologic patterns of lung disease. Use the clues to identify the pattern for each column, then complete the missing information.
| Name of pattern | Embolic pneumonia | Bronchopneumonia | Interstitial lung disease | Airway disease |
| Cause | Bacteria, fungi | Bacteria | Virus, sepsis, toxins, idiopathic | Viruses, bacteria, hypersensitivity |
| Route of exposure | Hematogenous | Airway | Usually hematogenous | Usually airway |
| Gross distribution of lesions | Generalized multifocal | Cranioventral | Generalized, diffuse (or sometimes, lobular) | Absent or inconsistent |
| Key histologic feature | Multiple random foci of inflammation | Inflammatory exudates fill bronchiolar and alveolar lumens | Hyaline membranes, type II pneumocyte proliferation, interstitial fibrosis | Necrosis of airway epithelial cells, or leukocyte infiltration targeting airways |
Answer:
List the two most important viral respiratory infections of cattle and of cats.
Cattle: Bovine herpesvirus-1 (IBR virus), bovine respiratory syncytial virus. BVDV predisposes to bacterial pneumonia but does not cause primary viral respiratory disease. Other viruses include coronavirus and parainfluenza virus.
Cats: Feline herpesvirus, feline calicivirus.
Answer:
List three mechanisms of airway obstruction in a pig with influenza.
- Bronchoconstriction.
- Edema thickening the airway wall due to inflammation.
- Necrotic epithelial cells and leukocytes partially fill the airway lumen.
Answer:
What is the difference between bronchiectasis and bronchiolitis obliterans, with respect to morphology and cause?
Bronchiectasis: permanent dilation of bronchi, caused by bacterial infection that results in pooling of exudates that obstructs and degrades the bronchial wall.
Bronchiolitis obliterans: fibrous polyp filling the lumen of a bronchiole, the result of prior viral infection.
Answer:
Give one example of a pneumoconiosis.
Pneumoconioses are chronic interstitial lung diseases caused by inhalation of inorganic dusts. Examples include asbestos, silica, coal dust.
Answer:
What are the two main features of chronic interstitial lung disease?
- Proliferation of type II pneumocytes
- Fibrosis of alveolar septa

