2 General pathology of the lung
This section deals with general pathologic processes in the lung, including hyperemia, congestion, hemorrhage, thrombosis, embolism, infarction, atelectasis, and emphysema.
Hyperemia
Hyperemia is increased blood flow, leading to red discoloration. Hyperemia occurs in inflammation. Look for other evidence of inflammation, such as the cranioventral distribution expected in bronchopneumonia, or the presence of inflammatory cells or fibrin.
Congestion
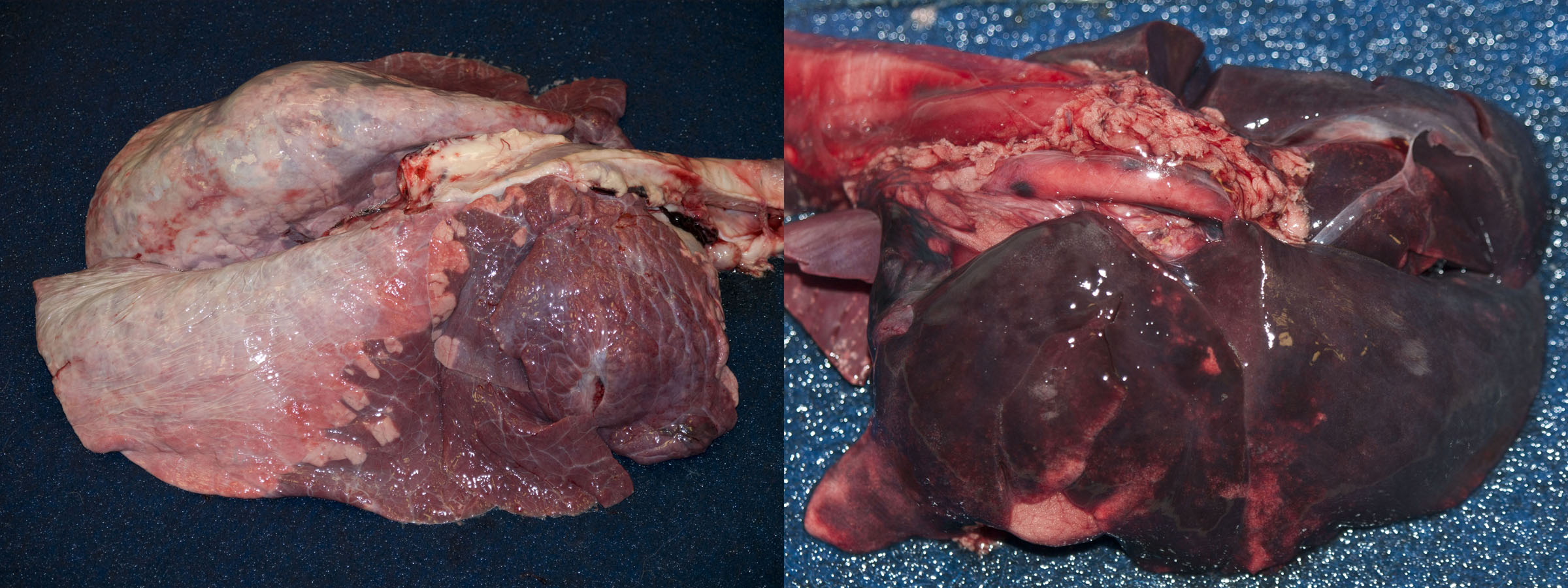
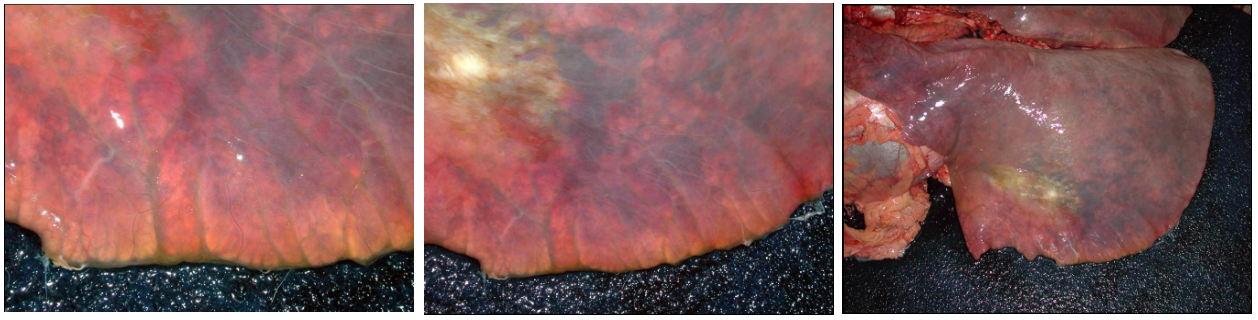
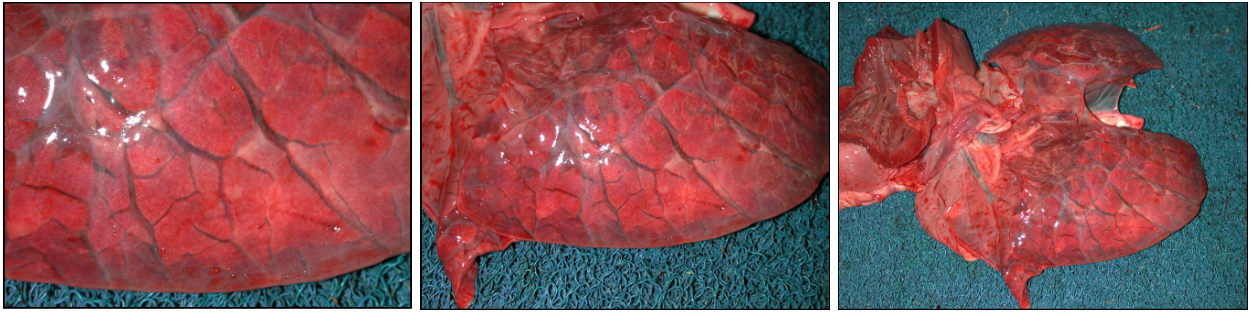
After death, venous blood often redistributes to the lungs. So, the lungs of dead animals are often red-purple instead of salmon-pink. This diffuse congestion should be interpreted with caution. However, if you see a localized area of red-purple lung, the colour change is a signpost. If the localized area of discoloration is also firmer on palpation, then it is probably an antemortem lesion.
Left heart failure causes pulmonary congestion: look for lesions in the heart.
Grossly, the congested lung is diffusely reddened, with no or little change in texture. In contrast, hemorrhage is usually multifocal or patchy, rather than diffuse. Hyperemia and congestion cannot be distinguished in dead tissues.
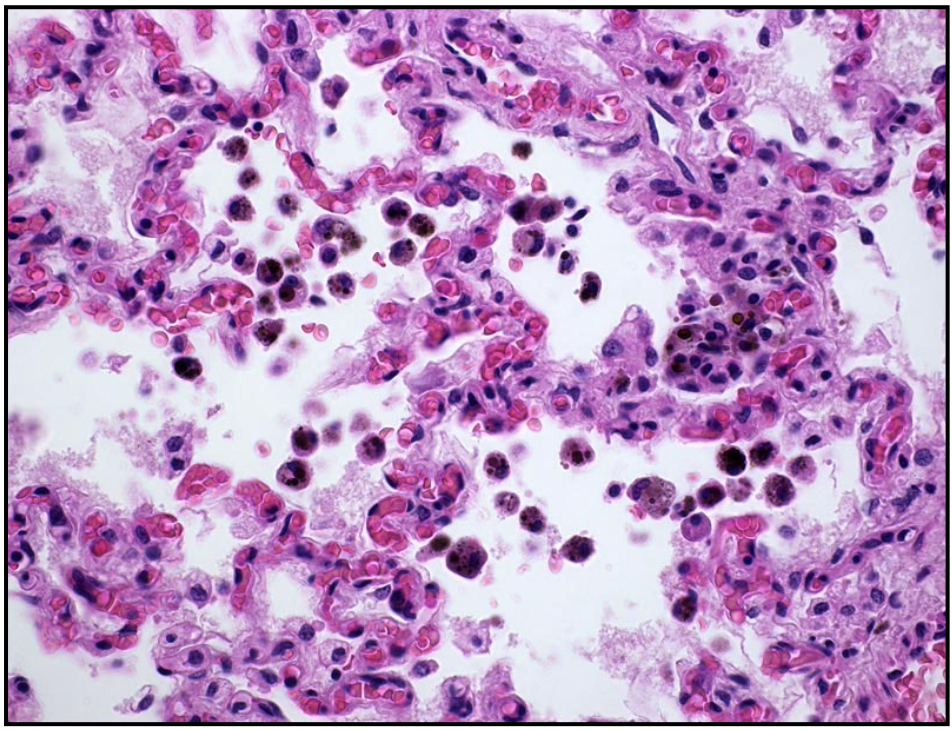
Histologically, severe congestion may lead to microscopic alveolar hemorrhage, with formation of hemosiderin-laden macrophages in the alveoli (“heart failure cells”).
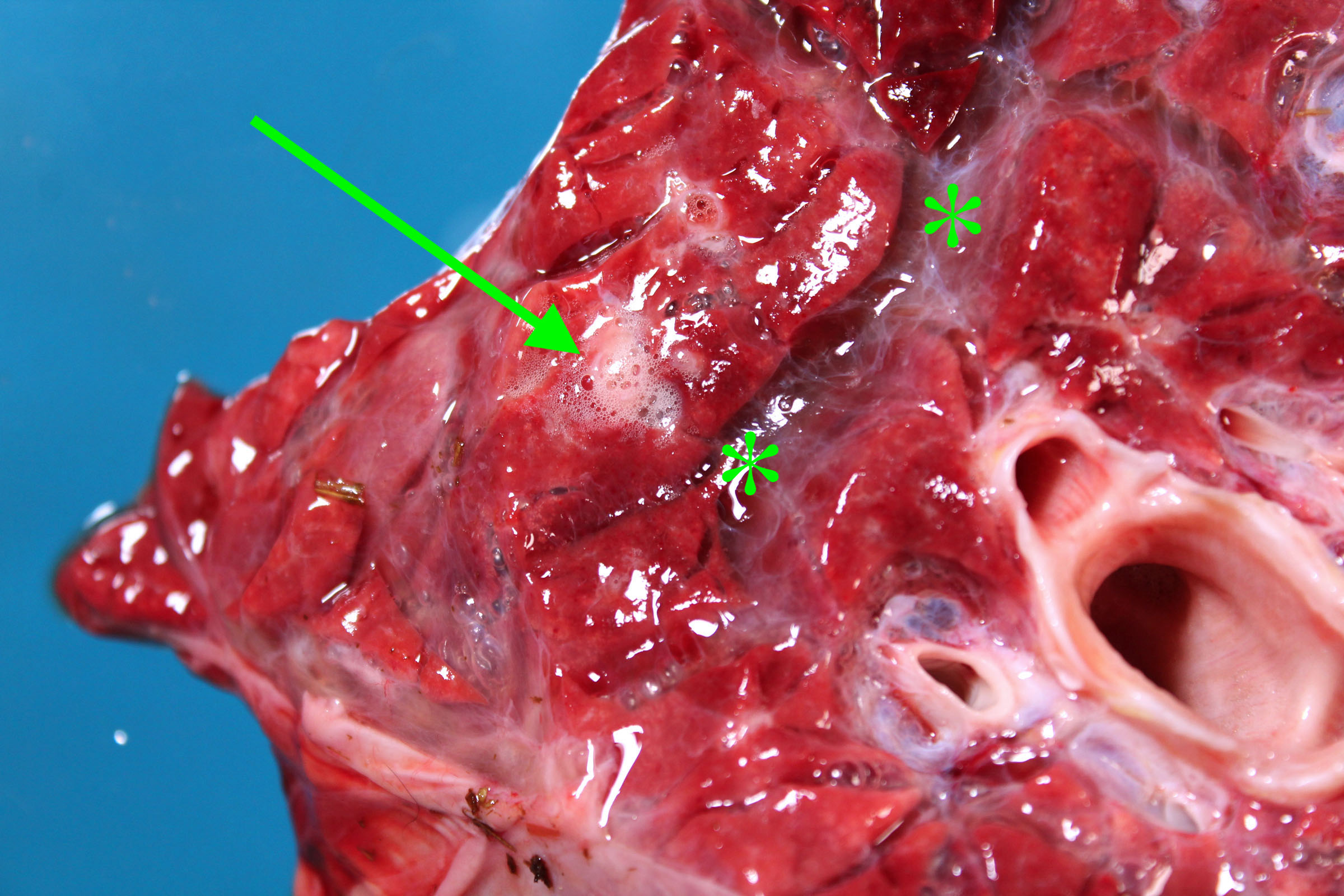
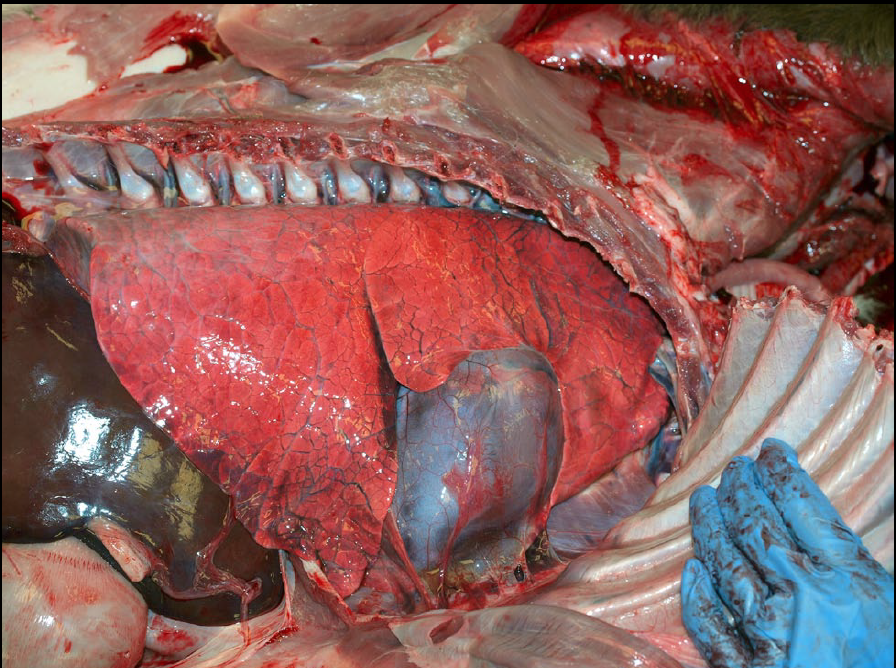
Edema
Mechanisms. There are 4 general mechanisms of edema in any tissue:
- Increased permeability of the blood-air barrier.
- As in other tissues, increased vascular permeability causes edema. This is most commonly the result of inflammation, such as in bronchopneumonia.
- Unique to the lung, the type I pneumocytes are also an important barrier to fluid movement from blood to alveolus. So in the lung, damage to either type I pneumocytes or to endothelial cells can both cause pulmonary edema. This is important in dogs with noncardiogenic pulmonary edema and in acute respiratory distress syndrome, and in infection with viruses that kill alveolar epithelial cells.
- The second category are diseases that damage endothelial cells or type I pneumocytes, and result in interstitial lung disease. The alveolar endothelium and epithelium are both important in keeping alveoli dry, and damage from viruses or from endotoxemia will lead to pulmonary edema.
- Increased venous pressure: left heart failure, or excessive administration of intravenous fluids.
- Lymphatic obstruction: masses/tumours, etc. This is an uncommon mechanism of pulmonary edema.
- Reduced oncotic pressure caused by hypoproteinemia (glomerular or intestinal disease). Although this is an important mechanism of edema in other organs, it usually does not cause edema in the lung.
Morphology. Grossly, edema is detected by a combination of the following:
- Heavy: water is heavier than air, so edema increases lung weight. Lift the lungs, to develop a sense of normal lung weights.
- Fluid leaks from the cut surface of the lung, or foam can be squeezed from the cut surface with gentle compression.
- Foam is abundant in the trachea and bronchi. But be cautious: some foam is normal after death, and is usually abundant in horses and sheep.
- Interlobular septa are more prominent than normal because they are separated by clear fluid. This is seen in ruminants, horses and pigs, but not in cats and dogs because they have less-developed interlobular septa.
Consequences. Edema fills alveoli and bronchioles, preventing ventilation of alveoli as well as diffusion of gases across the blood-air barrier in the alveoli. Less obviously, edema disrupts the surfactant layer in the alveoli, increasing the surface tension, and reducing lung compliance. Thus, in addition to the obvious effects on getting air into alveoli, more effort is needed to expand the edematous lung.
Question:
List the 3 most likely mechanisms of edema in the lung, and a likely specific cause for each. (Answers are provided at the end of the chapter).
Hemorrhage
Hemorrhage usually causes focal, multifocal, or patchy areas of red-purple discoloration and increased firmness of lung. In contrast, congestion of the lung from heart failure or as a postmortem change is diffuse or nearly diffuse. So, we mainly think of pulmonary hemorrhage when we see foci or “splashes” of dark red-purple lung tissue, not for a diffusely red-purple lung.
Common causes of pulmonary hemorrhage include exercise-induced pulmonary hemorrhage in racing horses, lung trauma (eg gunshot, or motor vehicle accidents) that lacerates blood vessels, and abscesses or tumours that bleed after they erode pulmonary blood vessels. Petechial or ecchymotic hemorrhages in the lung can result from thrombocytopenia, sepsis, or disseminated intravascular coagulation. In contrast, coagulation disorders such as anticoagulant rodenticide toxicity usually cause body-cavity hemorrhages (eg hemothorax), subcutaneous hematomas, or intra-articular hemorrhage, but not petechial hemorrhages.
The consequences of pulmonary hemorrhage usually relate more to blood loss, rather than compromised lung function.
Alveolar macrophages phagocytose erythrocytes, and hemoglobin is degraded to hemosiderin. Hemosiderin-laden macrophages persist in the alveoli, and are a histologic indicator of previous hemorrhage. These are referred to as “heart failure cells” because left-sided heart failure is one common cause, but hemosiderin-laden macrophages are also seen in other causes of pulmonary hemorrhage (such as exercise-induced pulmonary hemorrhage in horses).
Thrombosis, embolism, ischemia, infarction
Pulmonary thrombosis and embolism is an important cause of disease and sudden death in humans but is less commonly recognized in domestic animals. Because all blood from the right ventricle must pass through the lungs, obstruction of flow through the pulmonary system can have serious consequences.
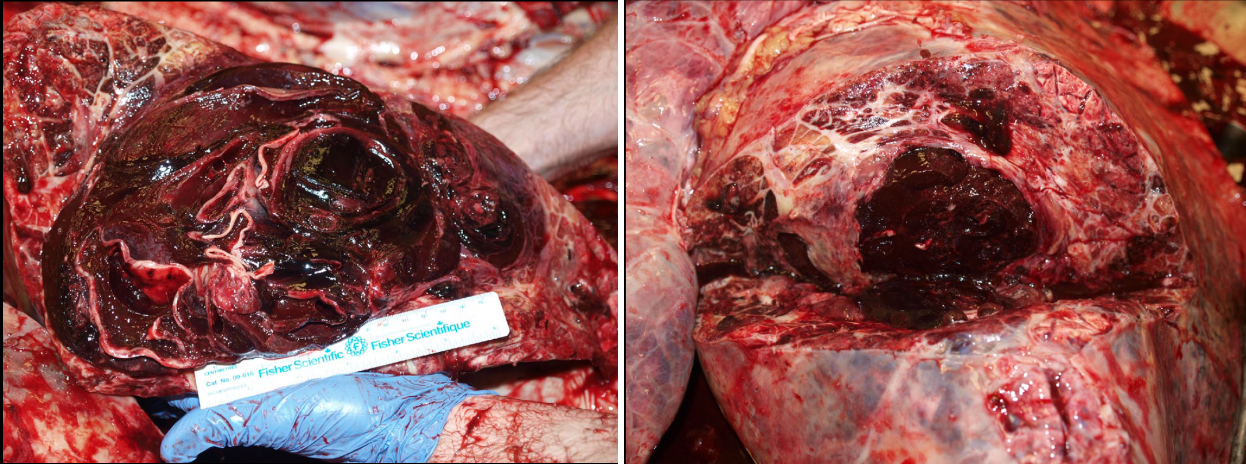
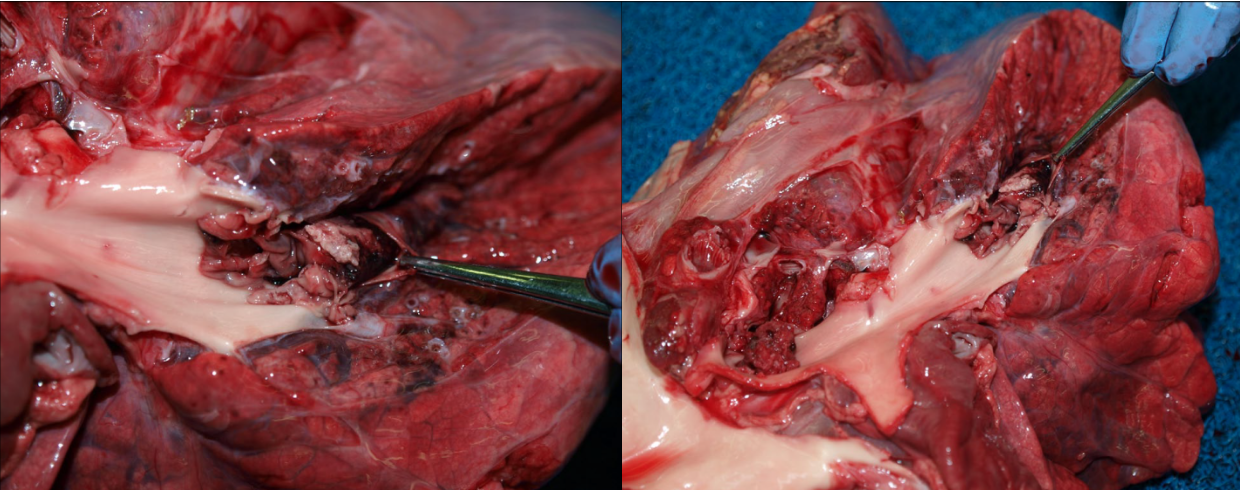
Thrombosis. Thrombi that form in situ in the lung are usually not grossly visible, whereas emboli may be grossly visible. Thrombi form in situ in the lung in hypercoagulable states: glomerular disease with loss of antithrombin, corticosteroid treatment or hyperadrenocorticism, disseminated intravascular coagulation, sepsis, or disseminated neoplasia.
Pulmonary embolism may originate from endocarditis of the right heart valves, from liver abscesses in cattle, from jugular thrombi as a complication of intravenous injection, and occasionally other unusual primary sites. They appear as tan, granular, non-elastic and often crumbly masses filling the lumen of a branch of the pulmonary arteries, in contrast to the red, elastic blood clots that are normally present. The pulmonary arteries should be opened in any case with respiratory signs, especially if gross examination doesn’t reveal an obvious or definite cause. If you don’t open the pulmonary arteries, you will miss an easy but important diagnosis. After you open the right heart and pulmonic valve, take the extra 15 seconds to extend this cut down the pulmonary arteries of the caudal lung lobes. Some day, you’ll be glad you did!
The functional importance of pulmonary thrombosis or embolism depends on the extent of vascular obstruction. Minor thrombosis or embolism may result in pulmonary hemorrhages that are of no functional consequence, because this area of lung may remain adequately perfused by bronchial arteries (systemic circulation). Ischemia and infarction of the lung are more likely if thrombosis or embolism occurs at the periphery of the lung, or if the bronchial or systemic circulation is also impaired. Emboli that obstruct a large proportion of the branches of the pulmonary artery may cause right heart failure or sudden death because of obstruction to flow.
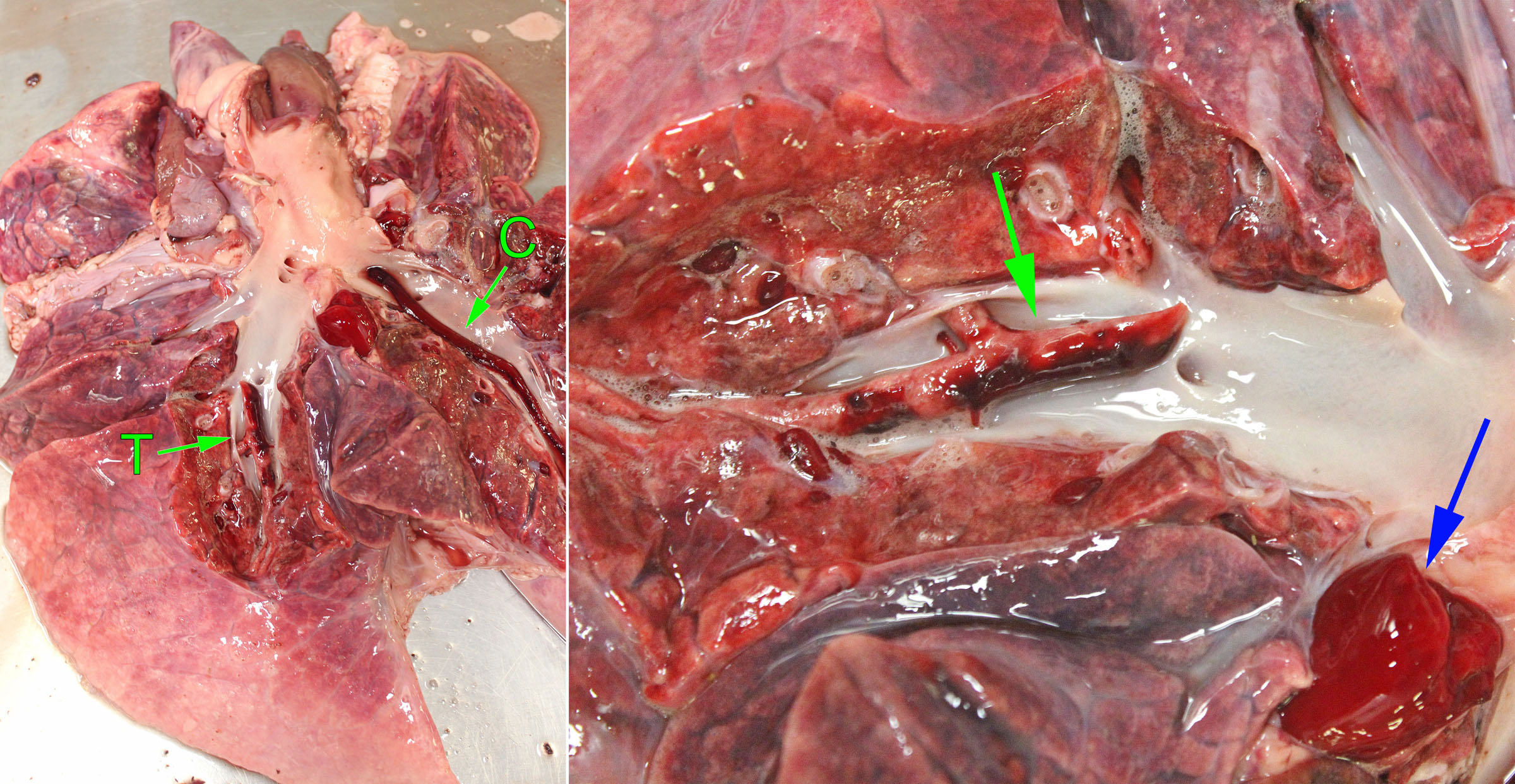
Question:
When you find a grossly visible thrombus in a large pulmonary artery, what are your considerations with respect to finding the cause? (Answers are provided at the end of the chapter).
Atelectasis
Definition: failure of the lung (alveoli) to expand / inflate.
Gross appearance: The entire lung, or the affected lobules, are collapsed compared to normal, is reddened because capillaries are more closely spaced, and is rubbery and lacks the spongy texture of normal lung because of the absence of air.
Causes
- Airway obstruction: air cannot enter alveoli, and trapped air is gradually absorbed. For example, in bronchiolar inflammation, neutrophils fill and obstruct the bronchioles, causing a lobular pattern of alveolar collapse.
- Compression of the lung: pleural effusions, pneumothorax, rumen tympany/bloat, diaphragmatic hernia.
- Surfactant dysfunction. Surfactant reduces surface tension in the lung and permits expansion of the alveoli. Animals that are born prematurely may not have produced surfactant yet, and their lungs may fail to inflate.
- Congenital atelectasis: lung inflates after birth, so atelectasis in aborted or stillborn animals is normal.
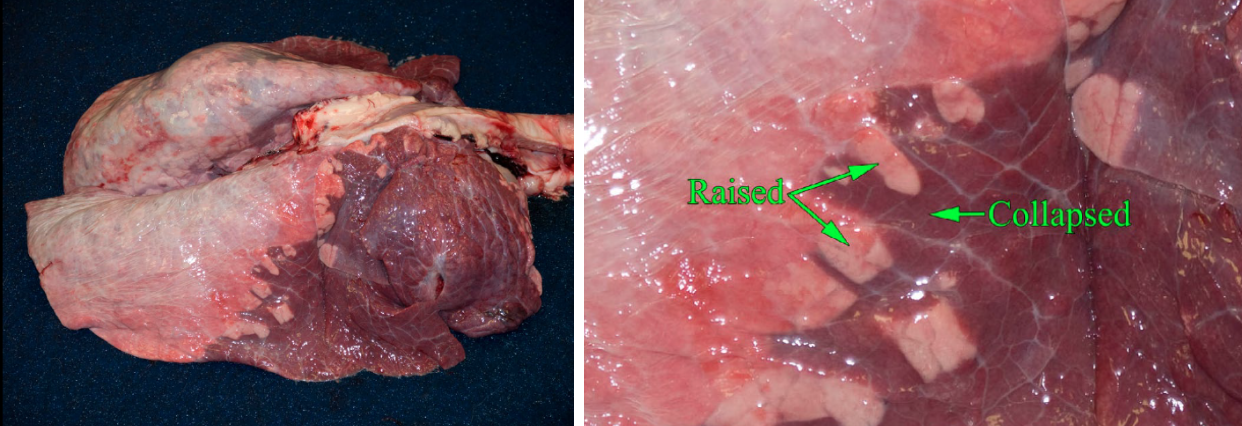
Question:
Explain how bronchiolar obstruction and pneumothorax can each cause atelectasis. (Answers are provided at the end of the chapter).
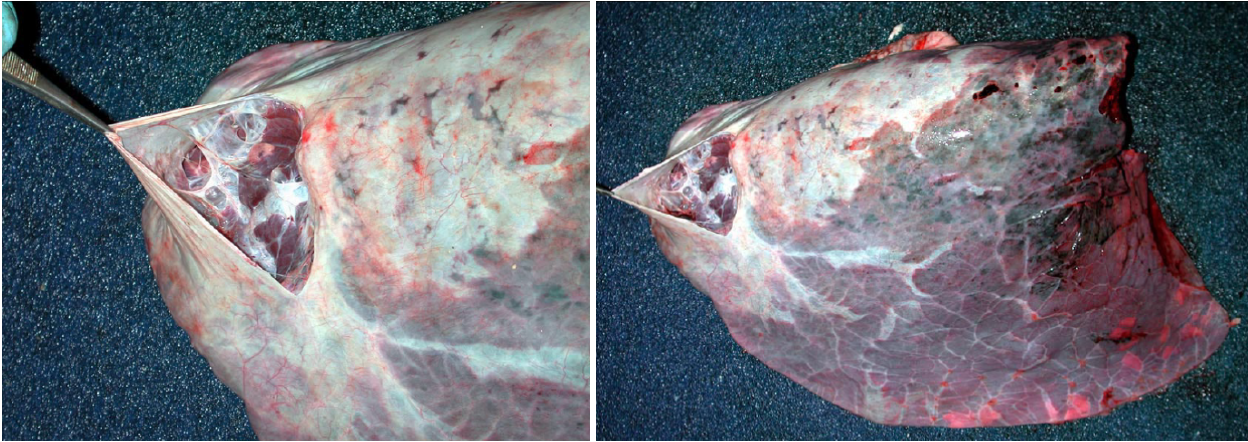
Emphysema and overinflation
- Overinflation: excessive air distending alveoli. This is reversible if the cause can be corrected.
- Alveolar emphysema: enlargement of alveolar airspaces due to destruction of alveolar walls.
- Interlobular and bullous emphysema: air bubbles within the interlobular septa or subpleural connective tissue.
Over-inflation or air trapping is common in animals. It is often the result of airway obstruction. Complete airway obstruction causes atelectasis. In contrast, with partial airway obstruction, gas can enter the lung when the airways are expanded during inhalation, but the gas is trapped in the alveoli when the airways collapse during exhalation. Bronchiolitis (e.g. viral infection, recurrent airway obstruction, or bacterial pneumonia) or masses such as tumours can cause air trapping in alveoli supplied by the affected airways.
Interlobular and bullous emphysema results from destruction of alveolar septa with subsequent loss of gas into the interlobular tissue. This is most often the result of severe dyspnea due to interstitial lung disease. It can also be seen in dyspneic animals with lung disease other than interstitial pneumonia, or even with non-respiratory disease such as downer cows with milk fever or toxic mastitis. Finally, barometric trauma can rupture alveoli; for example, positive pressure ventilation using excessive force, or accidental closure of the pop-off valve in the anesthetic circuit. Interlobular emphysema results in crepitus: a crackly texture when the lung is palpated. On the pleural surface or the cut section of lung, lines of air bubbles are seen, corresponding to the interlobular septa.
Alveolar emphysema is an important condition of human smokers but is rare in domestic animals. The enlargement of airspaces results from destruction of alveolar septa, which is a permanent change within the lung. Alveolar emphysema is detected grossly by an excessively spongy texture. A related condition in dogs is the formation of bulla (large air bubbles due to destruction of alveolar septa), which can rupture and cause spontaneous pneumothorax and severe dyspnea. The cause is unknown, but the great news is that most cases are cured by surgical removal of the affected lobe (lobectomy).

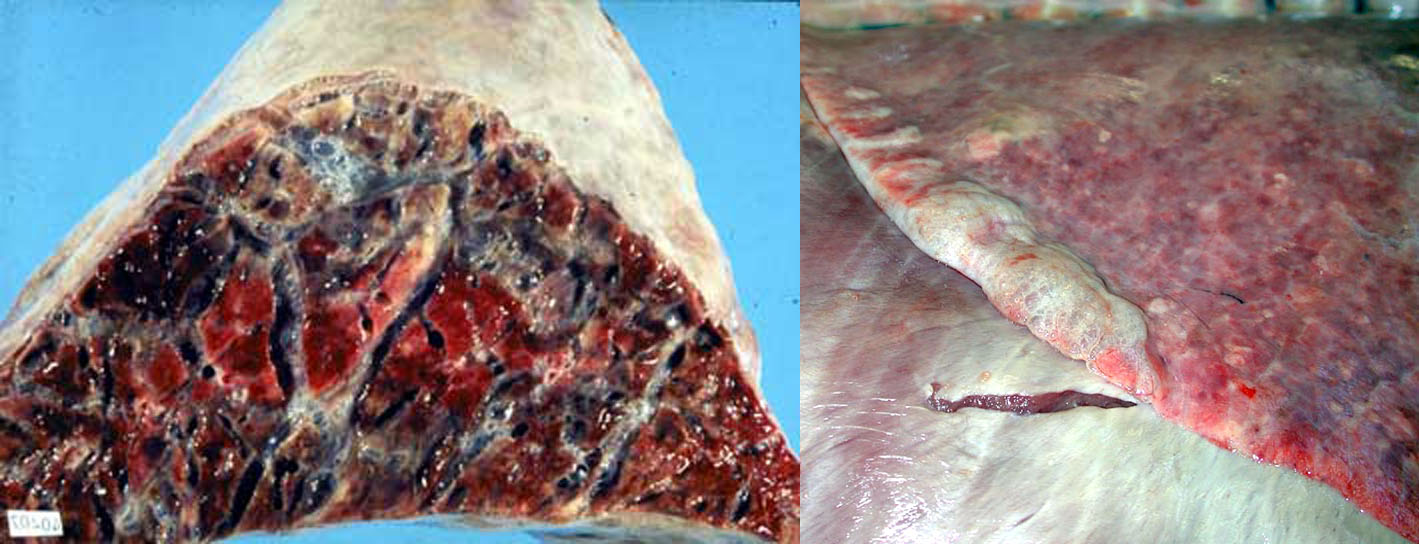
Question:
What is the clinical importance of distinguishing emphysema from air trapping in the alveolus? (Answers are provided at the end of the chapter).
Question:
List 2 reasons for increased prominence of interlobular septa. (Answers are provided at the end of the chapter).
pulmonary neoplasia
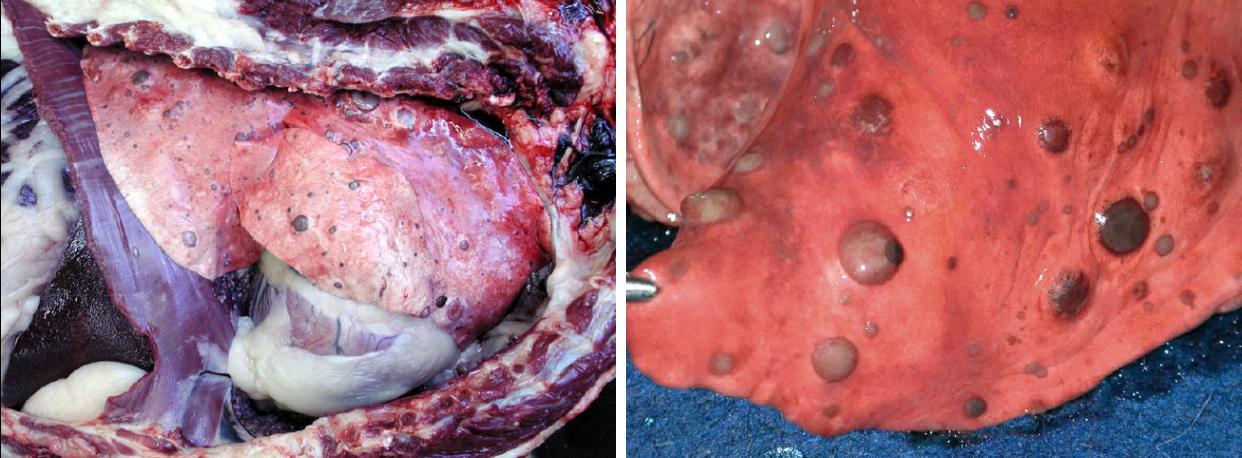
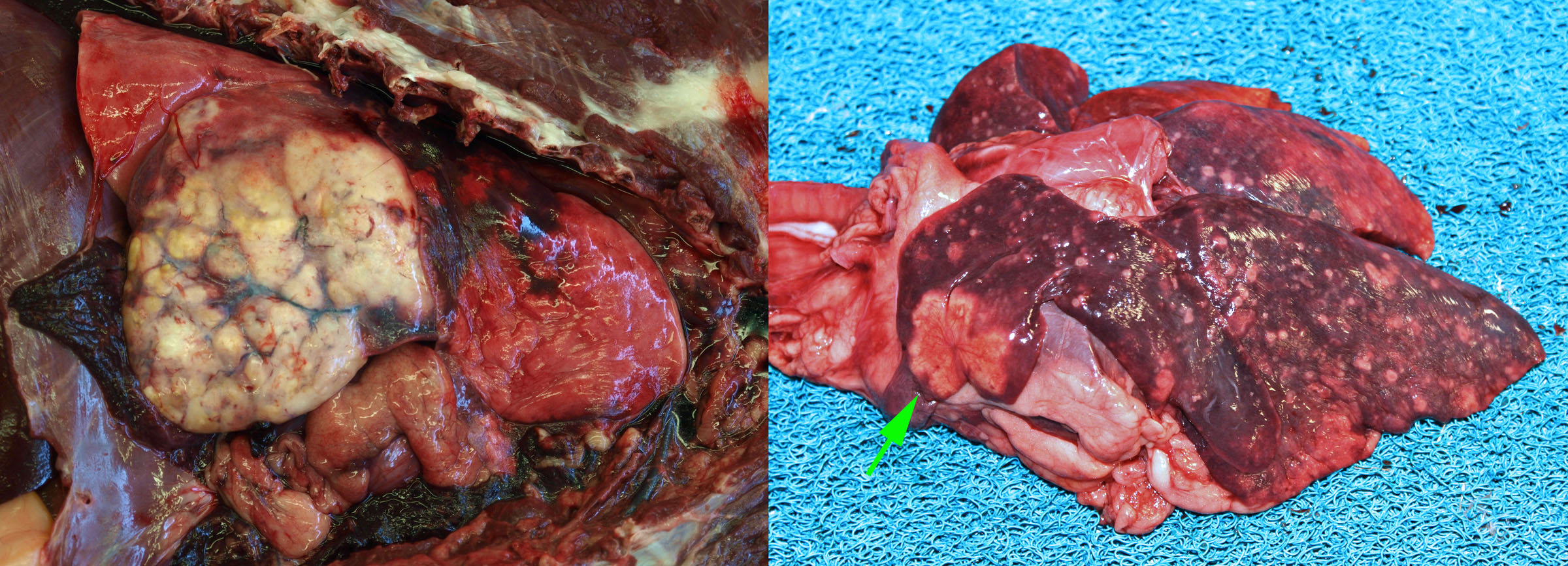
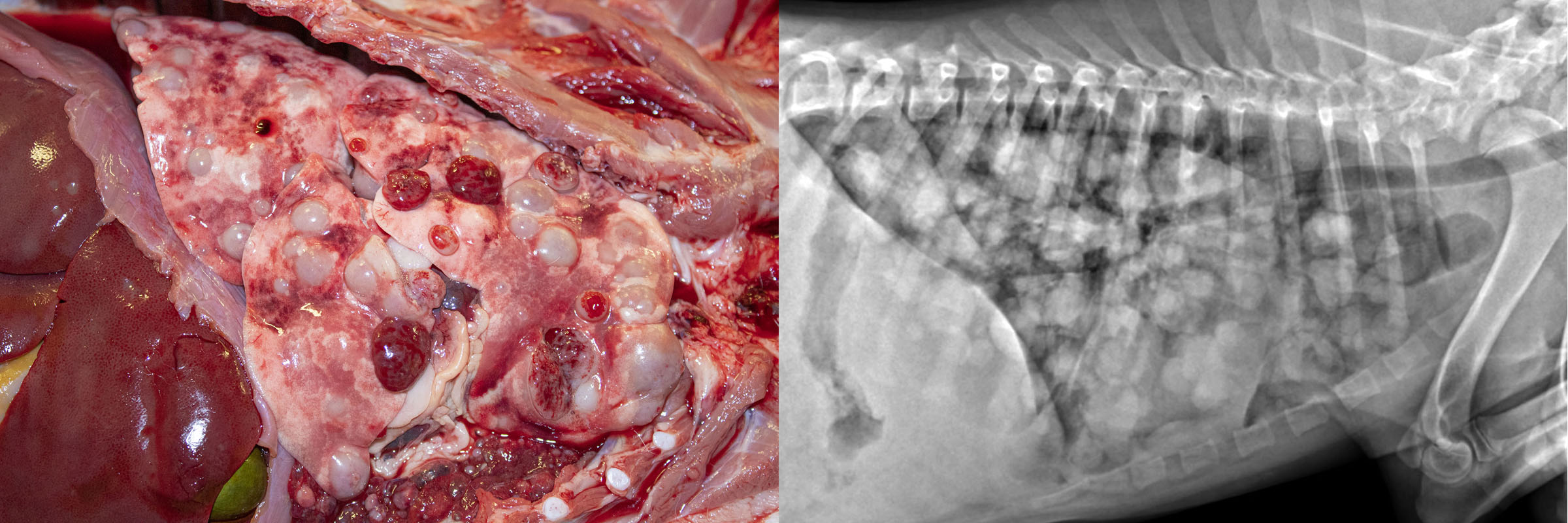
Most tumours affecting the lungs of domestic animals are metastases from a distant site. Primary lung tumours—those arising in the lung—are very important in humans, but uncommon in domestic animals.
Metastatic lung tumours form multiple small nodules throughout the lung. In contrast, primary lung tumours usually cause one or a few larger masses. Note that primary lung tumors may metastasize within the lung before venturing to more distant sites, and such cases typically have a large primary mass and multiple small metastases.
Pulmonary carcinoma is uncommon in dogs, and a little more common condition in cats. The condition in cats is unique because the metastases, not the lung tumour, are frequently responsible for the presenting clinical signs. Pulmonary adenocarcinoma in cats metastasizes to unusual places—footpad, skeletal muscle, bone, eye, or brain—and frequently invades through the pleura to cause pleural effusion.
The clinical manifestations of lung tumours result from:
- Lung failure from occupying space within the lung tissue
- Compressing an important structure such as the bronchus
- Invasion into the pleura, causing pleural effusion that leads to dyspnea
- Rupture leading to blood loss (hemangiosarcoma is a typical example)
- Metastasis (e.g. feline pulmonary carcinoma to the digit, described above)
- Systemic effects of malignancy: malaise, anorexia, weight loss
- Paraneoplastic syndrome: hypertrophic osteopathy, manifesting as periosteal new bone formation in the long bones
Question:
List 2 substantially different ways that lung tumours can cause cough. (Answers are provided at the end of the chapter).
Question:
List 2 ways to differentiate, at the time of necropsy, primary from metastatic neoplasia of the lung. (Answers are provided at the end of the chapter).
Question:
List 2 ways that a (primary) pulmonary carcinoma could cause distal limb lesions. (Answers are provided at the end of the chapter).
Question:
Is visual appearance or texture more reliable for gross assessment of lung? (Answers are provided at the end of the chapter).
Answer:
List the 3 most likely mechanisms of edema in the lung, and a likely specific cause for each.
- Increased hydrostatic/venous pressure, e.g. left heart failure.
- Reduced oncotic pressure, e.g. glomerular disease.
- Increased vascular permeability, e.g. bronchopneumonia or acute interstitial lung injury.
- Damage to type I pneumocytes, such as viral infection.
- (Lymphatic obstruction).
Answer:
When you find a grossly visible thrombus in a large pulmonary artery, what are your considerations with respect to finding the cause?
This is likely an embolism, whereas in situ thrombi are usually (but not always) microscopic. Look for sources of embolism: heart valves, jugular vein, liver/caudal vena cava, and elsewhere. Consider whether the thrombi are extensive enough to obstruct blood flow.
Answer:
Explain how bronchiolar obstruction and pneumothorax can each cause atelectasis.
Bronchiolar obstruction prevents ventilation of the alveoli. Once the trapped gas is absorbed, the alveoli collapse and cannot be re-filled.
Pneumothorax dissociates the lung from the rib cage. With air in the pleura, we can’t generate negative intrapleural pressure, which is necessary to inflate the lung.
Answer:
What is the clinical importance of distinguishing emphysema from air trapping in the alveolus?
Emphysema is a permanent change. Air trapping will return to normal, if the airway obstruction can be resolved.
Answer:
List 2 reasons for increased prominence of interlobular septa.
Fluid: interlobular edema.
Air bubbles: interlobular emphysema.
Answer:
List 2 substantially different ways that lung tumours can cause cough.
Impinge on and irritate a large bronchus.
Fluid leakage from the tumour resulting in pulmonary edema.
Answer:
List 2 ways to differentiate, at the time of necropsy, primary from metastatic neoplasia of the lung.
- Primary: 1 or a few large masses, ± multiple metastases. Metastatic: multiple similarly sized masses.
- Evidence of a primary tumour in another organ.
- Histopathology can be helpful, but don’t neglect the importance of the above gross findings!
Answer:
List 2 ways that a (primary) pulmonary carcinoma could cause distal limb lesions.
- Metastasis causing a focal mass in skeletal muscle or bone of the limb. This is seen in cats with pulmonary carcinoma.
- Hypertrophic osteopathy, causing diffuse thickening of the periosteum.
Answer:
Is visual appearance or texture more reliable for gross assessment of lung?
Both are necessary, but trust your palpation skills. The visual appearance can be deceiving.

