Basic Concepts of Perfusion and Renal Elimination
To understand the effects of various cardiovascular medications, it is important to first understand the basic anatomy and physiology of the cardiovascular and renal system.
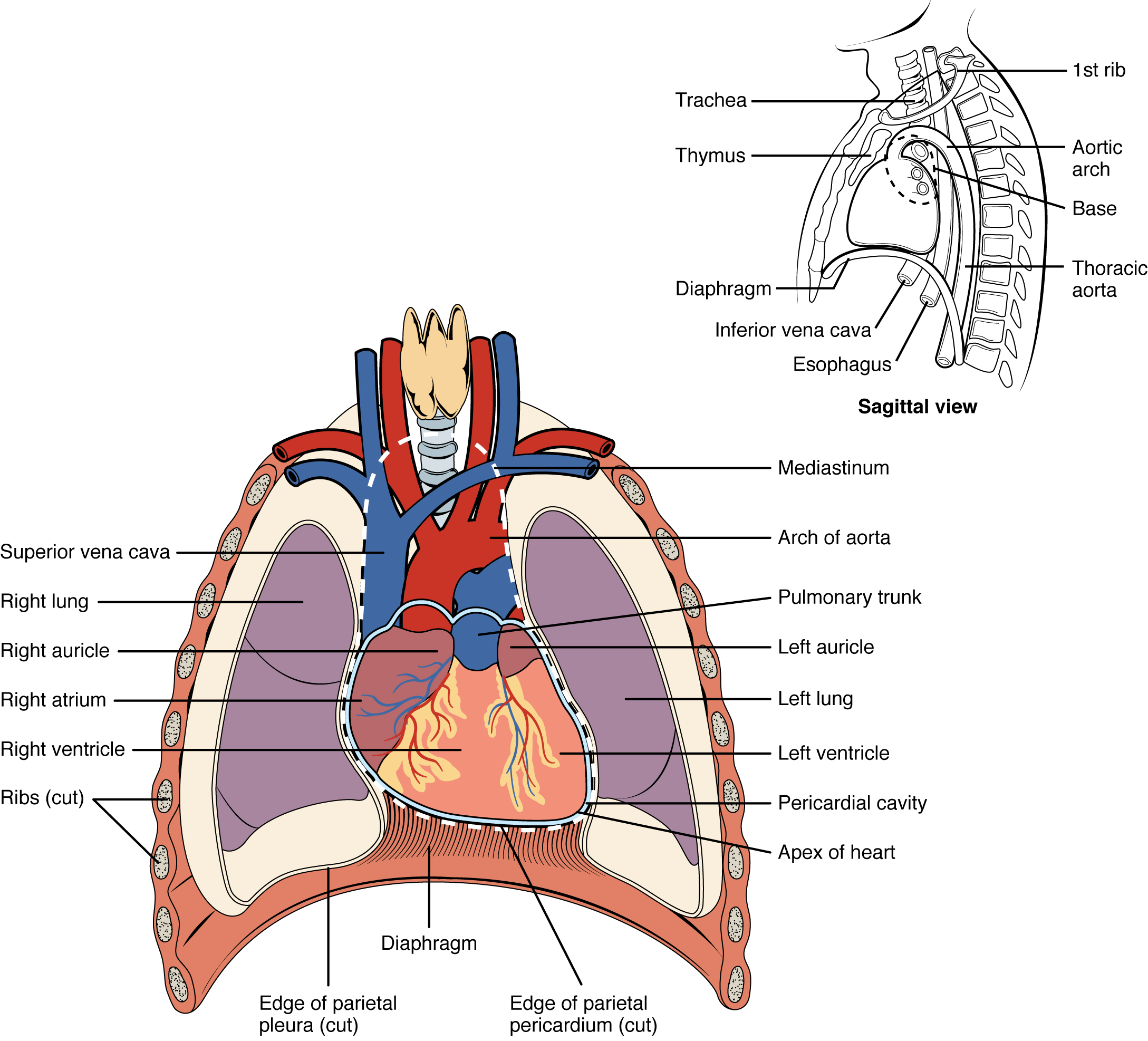
Location of the Heart
The human heart is located within the thoracic cavity, medially between the lungs in the space known as the mediastinum. The great veins, the superior and inferior venae cavae, and the great arteries, the aorta and pulmonary trunk, are attached to the superior surface of the heart, called the base. The base of the heart is located at the level of the third costal cartilage, as seen in Figure 6.2b.[1] The inferior tip of the heart, the apex, lies just to the left of the sternum between the junction of the fourth and fifth ribs. It is important to remember the position of the heart when placing a stethoscope on the chest of a client and listening for heart sounds.[2]
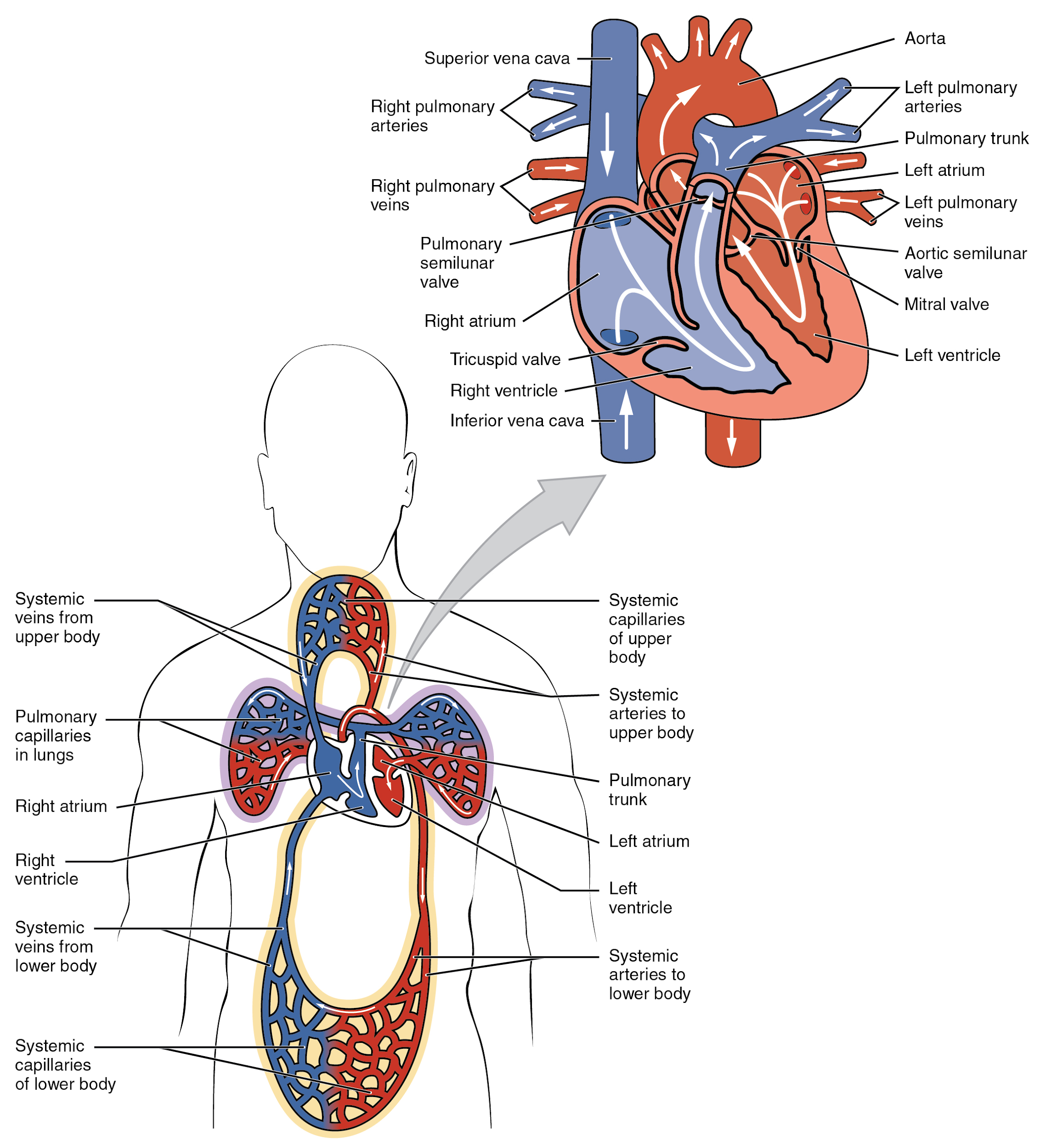
Chambers and Circulation through the Heart
The heart consists of four chambers: two atria and two ventricles. The right atrium receives deoxygenated blood from the systemic circulation, and the left atrium receives oxygenated blood from the lungs. The atria contract to push blood into the lower chambers, the right ventricle and the left ventricle. The right ventricle contracts to push blood into the lungs, and the left ventricle is the primary pump that propels blood to the rest of the body.
There are two distinct but linked circuits in the human circulation called the pulmonary and systemic circuits. The pulmonary circuit transports blood to and from the lungs, where it picks up oxygen and delivers carbon dioxide for exhalation. The systemic circuit transports oxygenated blood to virtually all of the tissues of the body and returns deoxygenated blood and carbon dioxide to the heart to be sent back to the pulmonary circulation. See Figure 6.2c[3] for an illustration of blood flow through the heart and blood circulation throughout the body.[4]
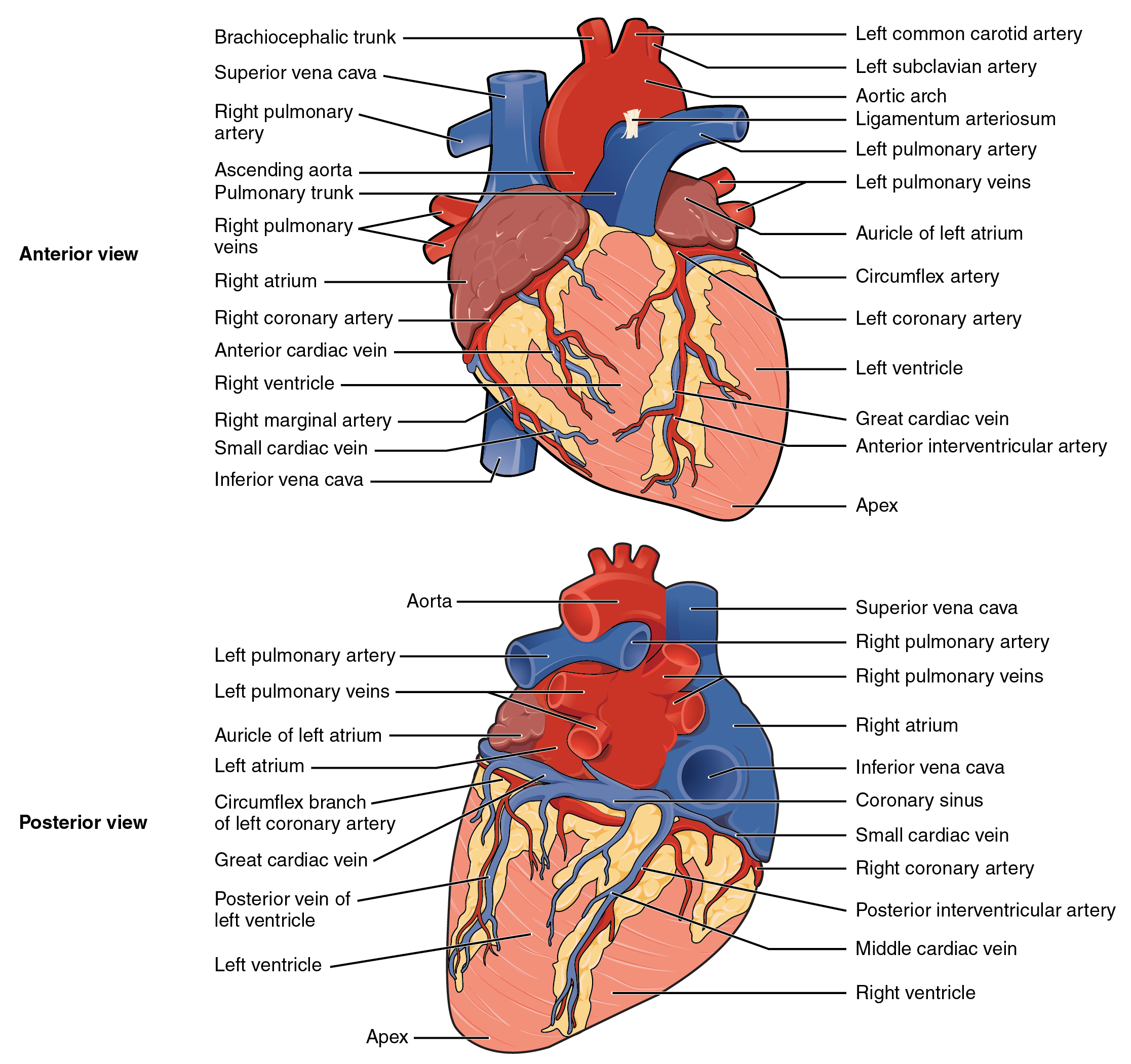
Blood also circulates through the coronary arteries with each beat of the heart. The left coronary artery distributes blood to the left side of the heart, and the right coronary distributes blood to the right atrium, portions of both ventricles, and the heart conduction system. See Figure 6.2d[5] for an illustration of the coronary arteries. When a client has a myocardial infarction, a blood clot lodges in one of these coronary arteries that perfuse the heart tissue. If a significant area of muscle tissue dies from lack of perfusion, the heart is no longer able to pump.
Conduction System of the Heart
Contractions of the heart are stimulated by the electrical conduction system. The components of the cardiac conduction system include the sinoatrial (SA) node, the atrioventricular (AV) node, the left and right bundle branches, and the Purkinje fibers. (See Figure 6.2e for an image of the conduction system of the heart.[6])
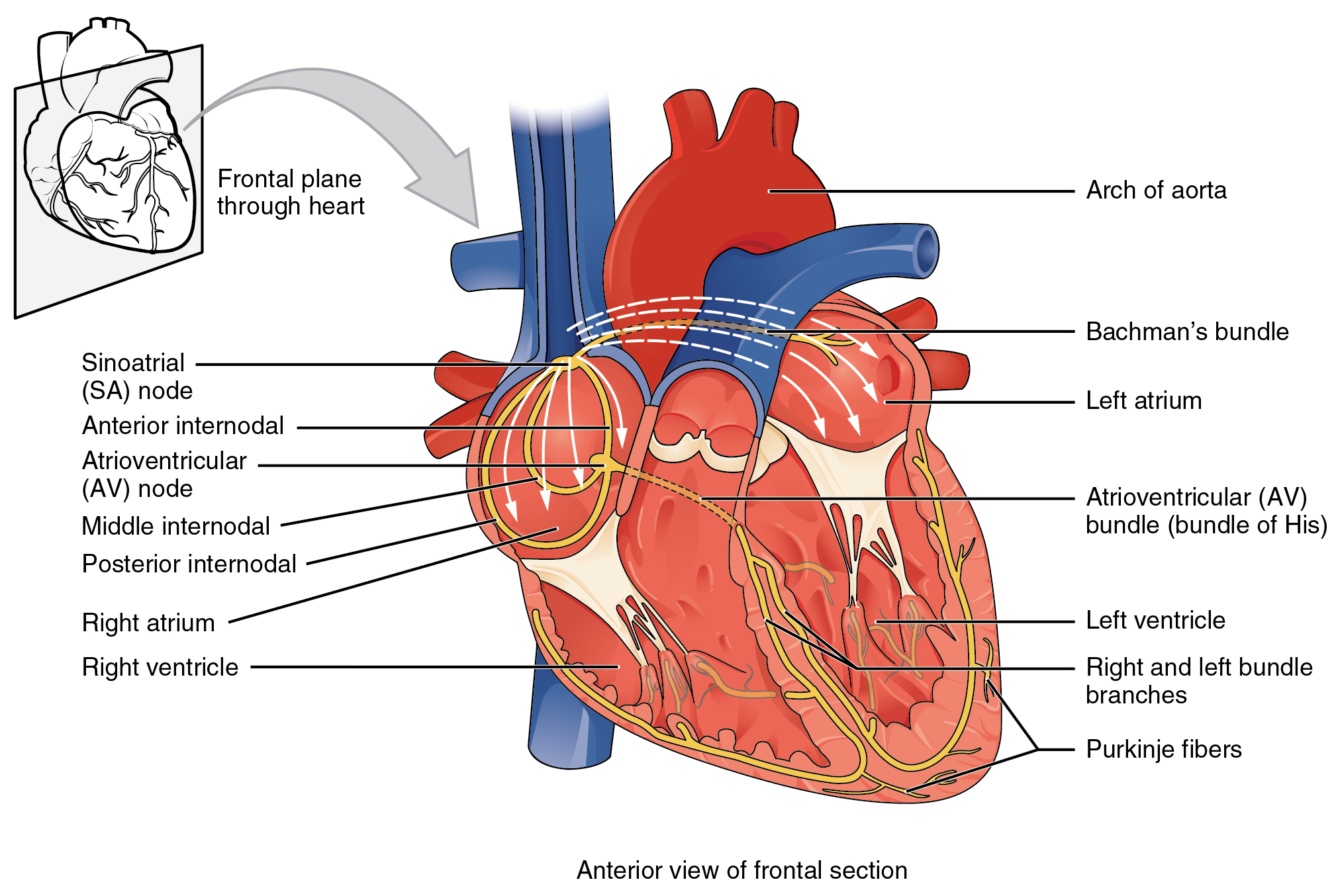
Normal cardiac rhythm is established by the sinoatrial (SA) node. The SA node has the highest rate of depolarization and is known as the pacemaker of the heart. Your SA node keeps our normal heart rate between 60-100 beats per minute. If there is damage to the SA node the AV node can take over pacing the heart, but this is at a substantially lower rate 40-60 beats per minute. Now if there is damage to both of these areas, the heart does have capacity to support itself with a rate of 20-40 beats per minute, but this impacts our cardiac output and appropriate functioning of the heart. We really want the SA node to function as it initiates the sinus rhythm or normal electrical pattern followed by contraction of the heart. The SA node initiates the action potential, which sweeps across the atria through the AV node to the bundle branches and Purkinje fibers, and then spreads to the contractile fibers of the ventricle to stimulate the contraction of the ventricle.[7]
Cardiac Conductive Cells
Sodium (Na), potassium (K) and calcium (Ca2) ions play critical roles in cardiac conducting cells in the conduction system of the heart. Unlike skeletal muscles and neurons, cardiac conductive cells do not have a stable resting potential. Conductive cells contain a series of sodium ion channels that allow influx of sodium ions that cause the membrane potential to rise slowly and eventually cause spontaneous depolarization. At this point, calcium ion channels open and Ca2 enters the cell, further depolarizing it. As the calcium ion channels then close, the K channels open, resulting in repolarization. When the membrane potential reaches approximately −60 mV, the K channels close and Na channels open, and the prepotential phase begins again. This phenomenon explains the autorhythmicity properties of cardiac muscle. Calcium ions play two critical roles in the physiology of cardiac muscle. In addition to depolarization, calcium ions also cause myosin to form cross bridges with the muscle cells that then provide the power stroke of contraction. Medications called calcium channel blockers thus affect both the conduction and contraction roles of calcium in the heart.
The autorhythmicity inherent in cardiac cells keeps the heart beating at a regular pace. However, the heart is regulated by other neural and endocrine controls, and it is sensitive to other factors, including electrolytes. These factors are further discussed in the homeostatic section below.[8]
Focus on Clinical Practice: The ECG
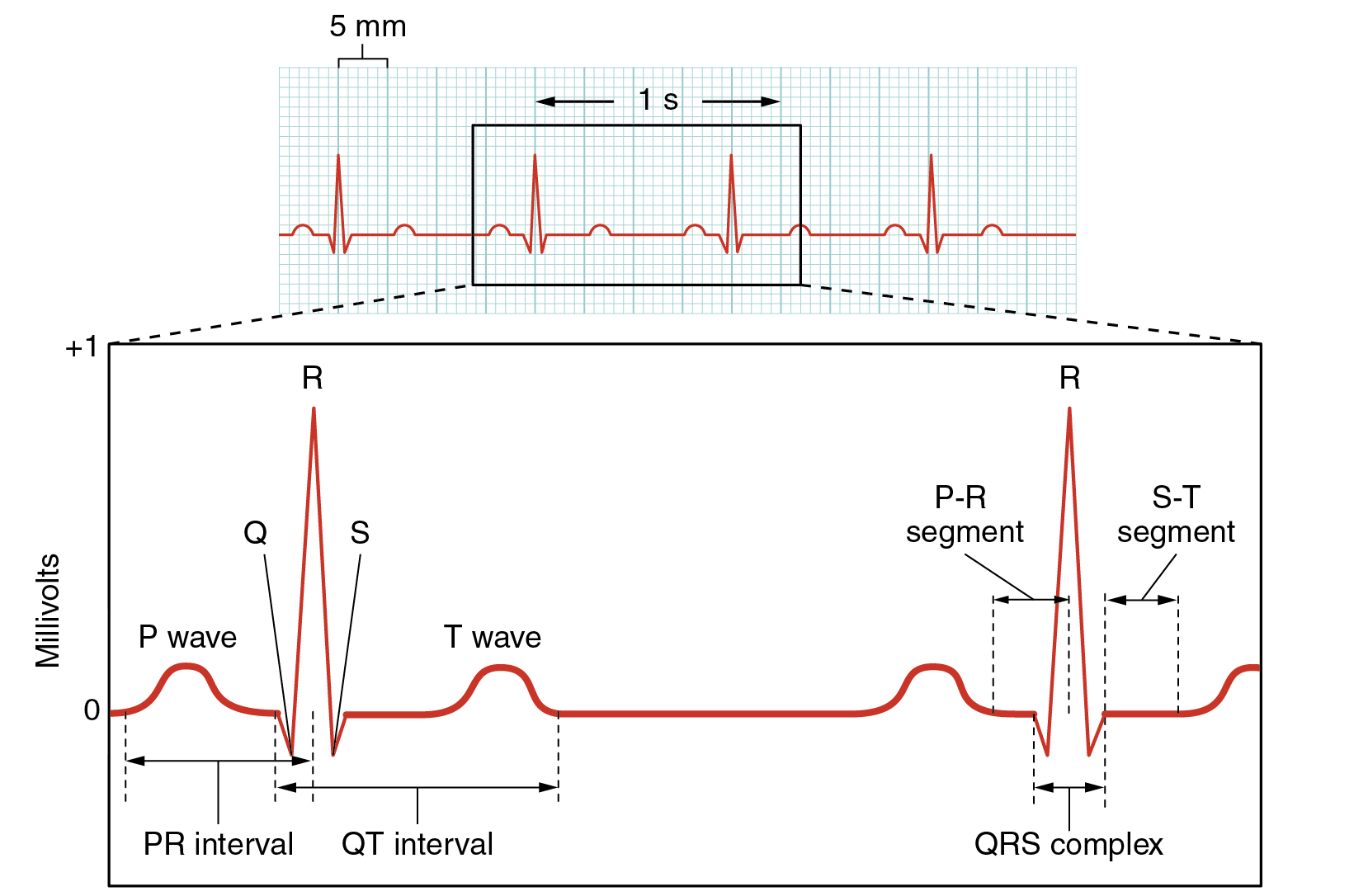
Surface electrodes placed on specific anatomical sites on the body can record the heart’s electrical signals. This tracing of the electrical signal is called an electrocardiogram (ECG), also historically abbreviated EKG. Careful analysis of the ECG reveals a detailed picture of both normal and abnormal heart function and is an indispensable clinical diagnostic tool. A normal ECG tracing is presented in Figure 6.2f[9]. Each component, segment, and the interval is labeled and corresponds to important electrical events.
There are five prominent components of the ECG: the P wave, the Q, R, and S components, and the T wave. The small P wave represents the depolarization of the atria. The large QRS complex represents the depolarization of the ventricles, which requires a much stronger impulse because of the larger size of the ventricular cardiac muscle. The ventricles begin to contract as the QRS reaches the peak of the R wave. Lastly, the T wave represents the repolarization of the ventricle. Several cardiac disorders can cause abnormal ECG readings called “dysrhythmias,” also called “arrhythmias,” and there are several types of antidysrhythmic medications used to treat these disorders that will be discussed later in this chapter.[10]
Cardiac Cycle
The period of time that begins with contraction of the atria and ends with ventricular relaxation is known as the cardiac cycle. The period of contraction that the heart undergoes while it pumps blood into circulation is called systole. The period of relaxation that occurs as the chambers fill with blood is called diastole.
Phases of the Cardiac Cycle
At the beginning of the cardiac cycle, both the atria and ventricles are relaxed (diastole). Blood is flowing into the right atrium from the superior and inferior venae cavae and into the left atrium from the four pulmonary veins. Contraction of the atria follows depolarization, which is represented by the P wave of the ECG. Just prior to atrial contraction, the ventricles contain approximately 130 mL blood in a resting adult. This volume is known as the end diastolic volume or preload. As the atrial muscles contract, pressure rises within the atria and blood is pumped into the ventricles.
Ventricular systole follows the depolarization of the ventricles and is represented by the QRS complex in the ECG. During the ventricular ejection phase, the contraction of the ventricular muscle causes blood to be pumped out of the heart. This quantity of blood is referred to as stroke volume (SV). Ventricular relaxation, or diastole, follows repolarization of the ventricles and is represented by the T wave of the ECG.[11]
Cardiac Output
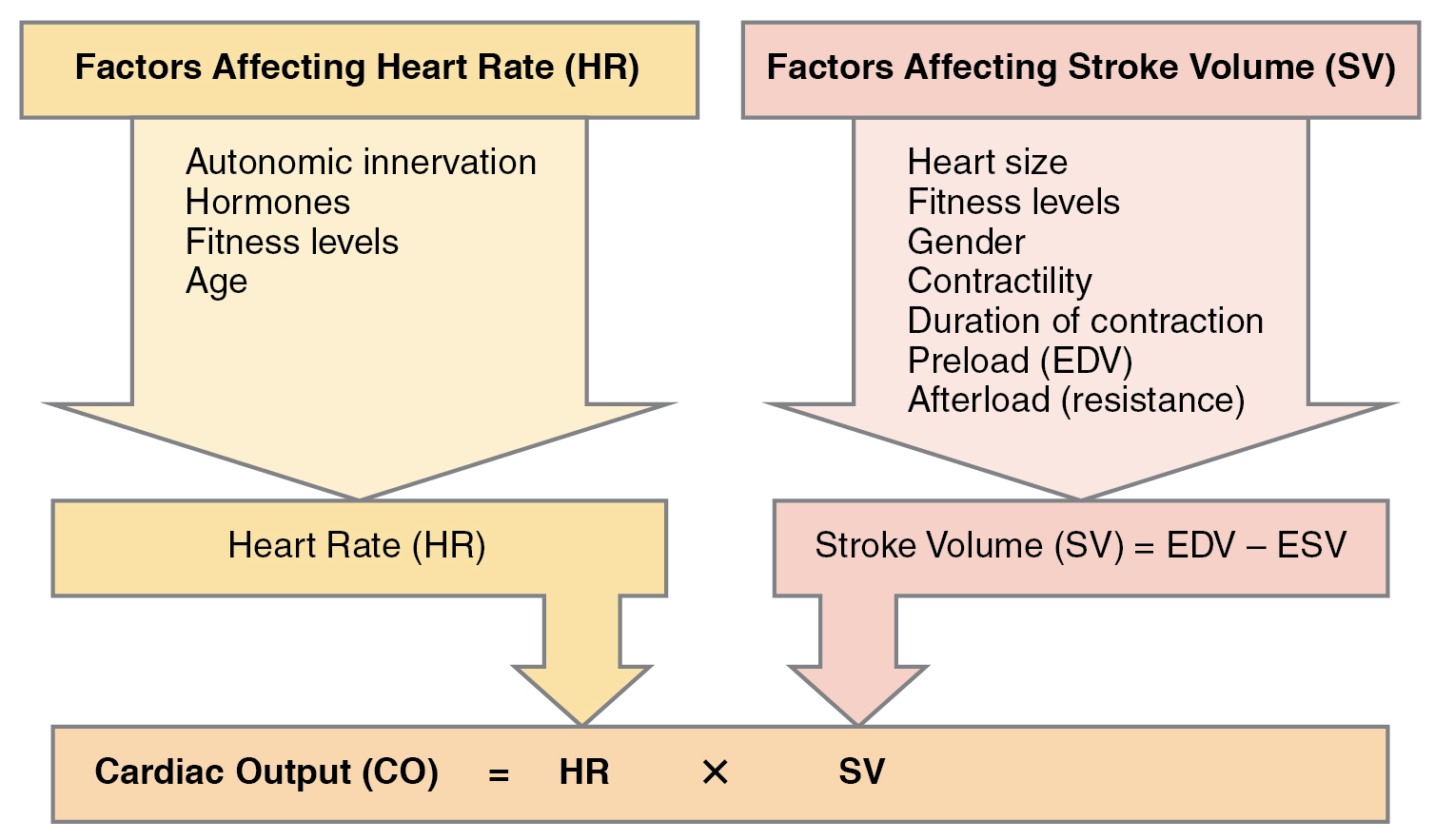
Cardiac output (CO) is a measurement of the amount of blood pumped by each ventricle in one minute. To calculate this value, multiply stroke volume (SV), the amount of blood pumped by each ventricle, by the heart rate (HR) in beats per minute. It can be represented mathematically by the following equation: CO = HR × SV. Factors influencing CO are summarized in Figure 6.2g[12] and include autonomic innervation by the sympathetic and parasympathetic nervous system, hormones such as epinephrine, preload, contractility, and afterload. Each of these factors is further discussed below.[13] SV is also used to calculate ejection fraction, which is the portion of the blood that is pumped or ejected from the heart with each contraction.
Heart Rate
Heart rate (HR) can vary considerably, not only with exercise and fitness levels, but also with age. Newborn resting HRs may be 120 -160 bpm. HR gradually decreases until young adulthood and then gradually increases again with age. For an adult, normal resting HR will be in the range of 60–100 bpm. Bradycardia is the condition in which resting rate drops below 60 bpm, and tachycardia is the condition in which the resting rate is above 100 bpm.
Correlation Between Heart Rates and Cardiac Output
Conditions that cause increased HR also trigger an initial increase in SV. However, as the HR rises, there is less time spent in diastole and, consequently, less time for the ventricles to fill with blood. As HR continues to increase, SV gradually decreases due to less filling time. In this manner, tachycardia will eventually cause decreased cardiac output.
Cardiovascular Centers
Sympathetic stimulation increases the heart rate and contractility, whereas parasympathetic stimulation decreases the heart rate. (See Figure 6.2h for an illustration of the ANS stimulation of the heart.[14]) Sympathetic stimulation causes the release of the neurotransmitter norepinephrine (NE), which shortens the repolarization period, thus speeding the rate of depolarization and contraction and increasing the HR. It also opens sodium and calcium ion channels, allowing an influx of positively charged ions.
NE binds to the Beta-1 receptor. Some cardiac medications (for example, beta blockers) work by blocking these receptors, thereby slowing HR and lowering blood pressure. However, an overdose of beta blockers can lead to bradycardia and even stop the heart.[15]
!["2032 Automatic Innervation.jpg" by OpenStax College is licensed under CC BY 4.0 Access for free at https://openstax.org/books/anatomy-and-physiology/pages/19-4-cardiac-physiology The vagus nerve (parasympathetic] decreases heart rate. Sympathetic cardiac nerves increases heart rate and force of contraction.](https://ecampusontario.pressbooks.pub/app/uploads/sites/3614/2023/10/image7-1.png)
Stroke Volume
Many of the same factors that regulate HR also impact cardiac function by altering SV. Three primary factors that affect stroke volume are: preload, or the stretch on the ventricles prior to contraction; contractility, or the force or strength of the contraction itself; and afterload, the force the ventricles must generate to pump blood against the resistance in the vessels. Many cardiovascular medications affect cardiac output by affecting preload, contractility, or afterload.[16]
Preload
Preload is another way of expressing end diastolic volume (EDV). Therefore, the greater the EDV is, the greater the preload is. One of the primary factors to consider is filling time, the duration of ventricular diastole during which filling occurs. Any sympathetic stimulation to the venous system will also increase venous return to the heart, which contributes to ventricular filling and preload. Medications such as diuretics decrease preload by causing the kidneys to excrete more water, thus decreasing blood volume.
Contractility
Contractility refers to the force of the contraction of the heart muscle, which controls SV. Factors that increase contractility are described as positive inotropic factors, and those that decrease contractility are described as negative inotropic factors.
Not surprisingly, sympathetic stimulation is a positive inotrope, whereas parasympathetic stimulation is a negative inotrope. The drug digoxin is used to lower HR and increase the strength of the contraction. It works by inhibiting the activity of an enzyme (ATPase) that controls movement of calcium, sodium, and potassium into heart muscle. Inhibiting ATPase increases calcium in heart muscle and, therefore, increases the force of heart contractions.
Negative inotropic agents include hypoxia, acidosis, hyperkalemia, and a variety of medications such as beta blockers and calcium channel blockers.
Afterload
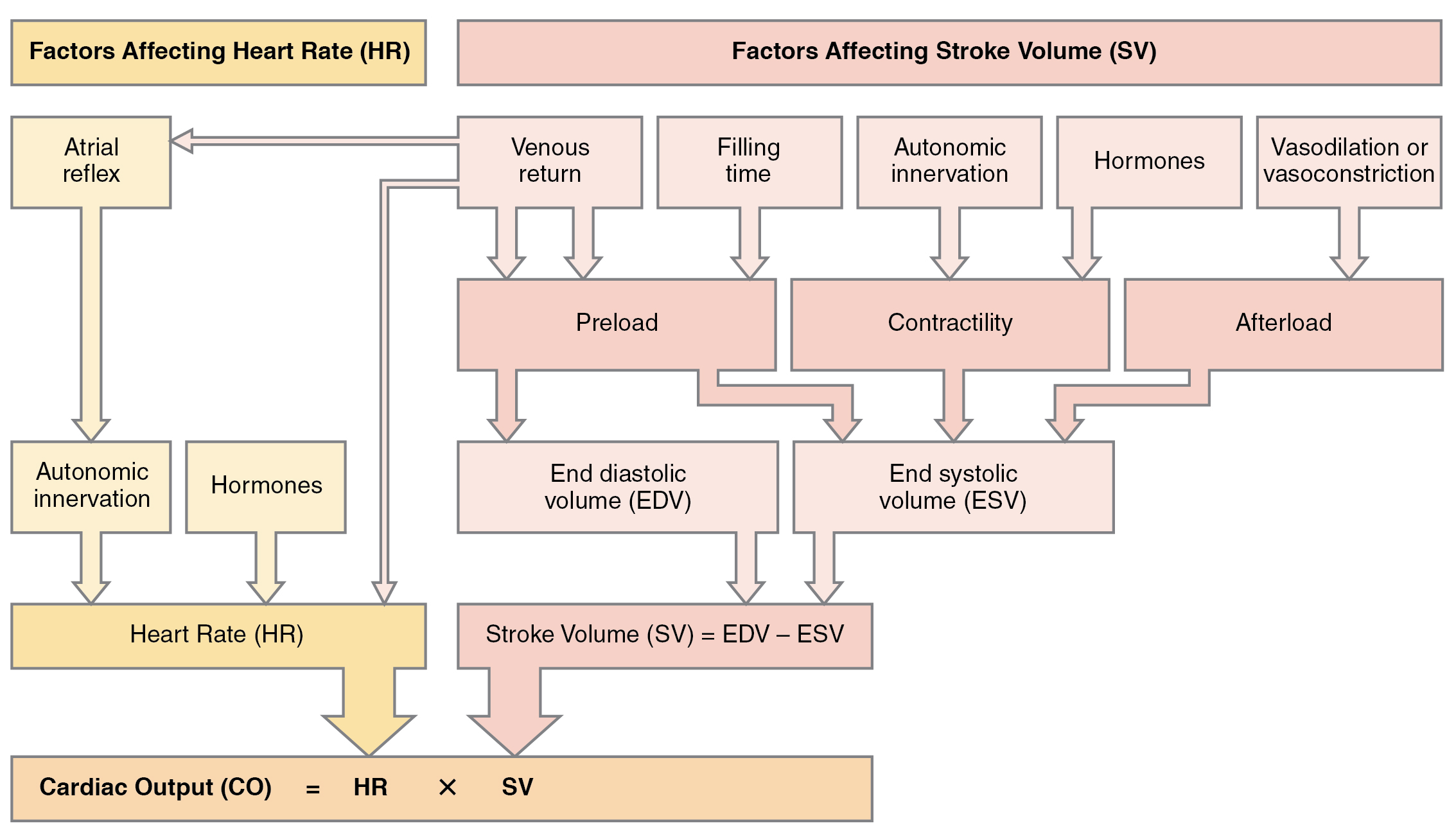
Afterload refers to the force that the ventricles must develop to pump blood effectively against the resistance in the vascular system. Any condition that increases resistance requires a greater afterload to force open the semilunar valves and pump the blood, which decreases cardiac output. On the other hand, any decrease in resistance reduces the afterload and thus increases cardiac output. Figure 6.2i[17] summarizes the major factors influencing cardiac output. Calcium channel blockers such as amlodipine, verapamil, nifedipine, and diltiazem can be used to reduce afterload and thus increase cardiac output.[18]
Systemic Circulation: Blood Vessels
After blood is pumped out of the ventricles, it is carried through the body via blood vessels. An artery is a blood vessel that carries blood away from the heart, where it branches into ever-smaller vessels and eventually into tiny capillaries where nutrients and wastes are exchanged at the cellular level. Capillaries then combine with other small blood vessels that carry blood to a vein, a larger blood vessel that returns blood to the heart. Compared to arteries, veins are thin-walled, low-pressure vessels. Larger veins are also equipped with valves that promote the unidirectional flow of blood toward the heart and prevent backflow caused by the inherent low blood pressure in veins as well as the pull of gravity.
In addition to their primary function of returning blood to the heart, veins may be considered blood reservoirs because systemic veins contain approximately 64 percent of the blood volume at any given time. Approximately 21 percent of the venous blood is located in venous networks within the liver, bone marrow, and integument. This volume of blood is referred to as venous reserve. Through venoconstriction, this reserve volume of blood can get back to the heart more quickly for redistribution to other parts of the circulation.
Nitroglycerin is an example of a medication that causes arterial and venous vasodilation. It is used for clients with angina to decrease cardiac workload and increase the amount of oxygen available to the heart. By causing vasodilation of the veins, nitroglycerin decreases the amount of blood returned to the heart, and thus decreases preload. It also reduces afterload by causing vasodilation of the arteries and reducing peripheral vascular resistance.[19]
Edema
Despite the presence of valves within larger veins, over the course of a day, some blood will inevitably pool in the lower limbs, due to the pull of gravity. Any blood that accumulates in a vein will increase the pressure within it. Increased pressure will promote the flow of fluids out of the capillaries and into the interstitial fluid. The presence of excess tissue fluid around the cells leads to a condition called edema. See Figure 6.2j[20] for an image of a client with pitting edema.
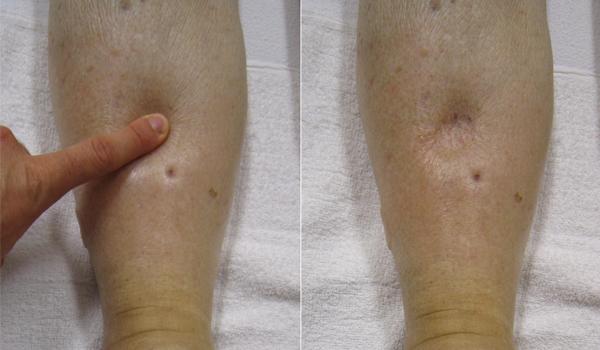
Most people experience a daily accumulation of fluid in their tissues, especially if they spend much of their time on their feet (like most health professionals). However, clinical edema goes beyond normal swelling and requires medical treatment. Edema has many potential causes, including hypertension and heart failure, severe protein deficiency, and renal failure. Diuretics such as furosemide are used to treat edema by causing the kidneys to eliminate sodium and water.[21]
Blood Flow and Blood Pressure
Blood flow refers to the movement of blood through a vessel, tissue, or organ. Blood pressure is the force exerted by blood on the walls of the blood vessels. In clinical practice, this pressure is measured in mm Hg and is typically obtained using a sphygmomanometer (a blood pressure cuff) on the brachial artery of the arm. When systemic arterial blood pressure is measured, it is recorded as a ratio of two numbers expressed as systolic pressure over diastolic pressure (e.g., 120/80 is a normal adult blood pressure). The systolic pressure is the higher value (typically around 120 mm Hg) and reflects the arterial pressure resulting from the ejection of blood during ventricular contraction or systole. The diastolic pressure is the lower value (usually about 80 mm Hg) and represents the arterial pressure of blood during ventricular relaxation or diastole.
Three primary variables influence blood flow and blood pressure:
- Cardiac output
- Compliance
- Volume of the blood
Any factor that causes cardiac output to increase will elevate blood pressure and promote blood flow. Conversely, any factor that decreases cardiac output will decrease blood flow and blood pressure. See the previous section on cardiac output for more information about factors that affect cardiac output.
Compliance is the ability of any compartment to expand to accommodate increased content. A metal pipe, for example, is not compliant, whereas a balloon is. The greater the compliance of an artery, the more effectively it is able to expand to accommodate surges in blood flow without increased resistance or blood pressure. When vascular disease causes stiffening of arteries, called arteriosclerosis, compliance is reduced and resistance to blood flow is increased. The result is higher blood pressure within the vessel and reduced blood flow. Arteriosclerosis is a common cardiovascular disorder that is a leading cause of hypertension and coronary heart disease because it causes the heart to work harder to generate a pressure great enough to overcome the resistance.
There is a relationship between blood volume, blood pressure, and blood flow. As an example, water may merely trickle along a creek bed in a dry season, but rush quickly and under great pressure after a heavy rain. Similarly, as blood volume decreases, blood pressure and flow decrease, but when blood volume increases, blood pressure and flow increase.
Low blood volume, called hypovolemia, may be caused by bleeding, dehydration, vomiting, severe burns, or by diuretics used to treat hypertension. Treatment typically includes intravenous fluid replacement. Excessive fluid volume, called hypervolemia, is caused by retention of water and sodium, as seen in clients with heart failure, liver cirrhosis, and some forms of kidney disease. Treatment may include the use of diuretics that cause the kidneys to eliminate sodium and water.[22]
Homeostatic Regulation of the Cardiovascular System
To maintain homeostasis in the cardiovascular system and provide adequate blood to the tissues, blood flow must be redirected continually to the tissues as they become more active. For example, when an individual is exercising, more blood will be directed to skeletal muscles, the heart, and the lungs. On the other hand, following a meal, more blood is directed to the digestive system. Only the brain receives a constant supply of blood regardless of rest or activity. Three homeostatic mechanisms ensure adequate blood flow and ultimately perfusion of tissues: neural, endocrine, and autoregulatory mechanisms.
Neural Regulation
The nervous system plays a critical role in the regulation of vascular homeostasis based on baroreceptors and chemoreceptors. Baroreceptors are specialized stretch receptors located within the aorta and carotid arteries that respond to the degree of stretch caused by the presence of blood and then send impulses to the cardiovascular center to regulate blood pressure. In addition to the baroreceptors, chemoreceptors monitor levels of oxygen, carbon dioxide, and hydrogen ions (pH). When the cardiovascular center in the brain receives this input, it triggers a reflex that maintains homeostasis.
Endocrine Regulation
Endocrine control over the cardiovascular system involves catecholamines, epinephrine, and norepinephrine, as well as several hormones that interact with the kidneys in the regulation of blood volume.
Epinephrine and Norepinephrine
The catecholamines epinephrine and norepinephrine are released by the adrenal medulla and are a part of the body’s sympathetic or fight-or-flight response. They increase heart rate and force of contraction, while temporarily constricting blood vessels to organs not essential for fight-or-flight responses and redirecting blood flow to the liver, muscles, and heart.
Antidiuretic Hormone
Antidiuretic hormone (ADH), also known as vasopressin, is secreted by the hypothalamus. The primary trigger prompting the hypothalamus to release ADH is increasing osmolarity of tissue fluid, usually in response to significant loss of blood volume. ADH signals its target cells in the kidneys to reabsorb more water, thus preventing the loss of additional fluid in the urine. This will increase overall fluid levels and help restore blood volume and pressure.
Renin-Angiotensin-Aldosterone System
The renin-angiotensin-aldosterone system (RAAS) has a major effect on the cardiovascular system. Specialized cells in the kidneys respond to decreased blood flow by secreting renin into the blood. Renin converts the plasma protein angiotensinogen into its active form—Angiotensin I. Angiotensin I circulates in the blood and is then converted into Angiotensin II in the lungs. This reaction is catalyzed by the enzyme called angiotensin-converting enzyme (ACE). Medications called ACE inhibitors such as lisinopril target this step in the RAAS in an effort to decrease blood pressure.
Angiotensin II is a powerful vasoconstrictor that greatly increases blood pressure. It also stimulates the release of ADH and aldosterone, a hormone produced by the adrenal cortex. Aldosterone then increases the reabsorption of sodium into the blood by the kidneys. Because water follows sodium, there is an increase in the reabsorption of water, which increases blood volume and blood pressure. See Figure 6.2k for an illustration of the renin-angiotensin-aldosterone system and Figure 6.2l[23] for a summary of the effect of hormones involved in renal control of blood pressure.[24]
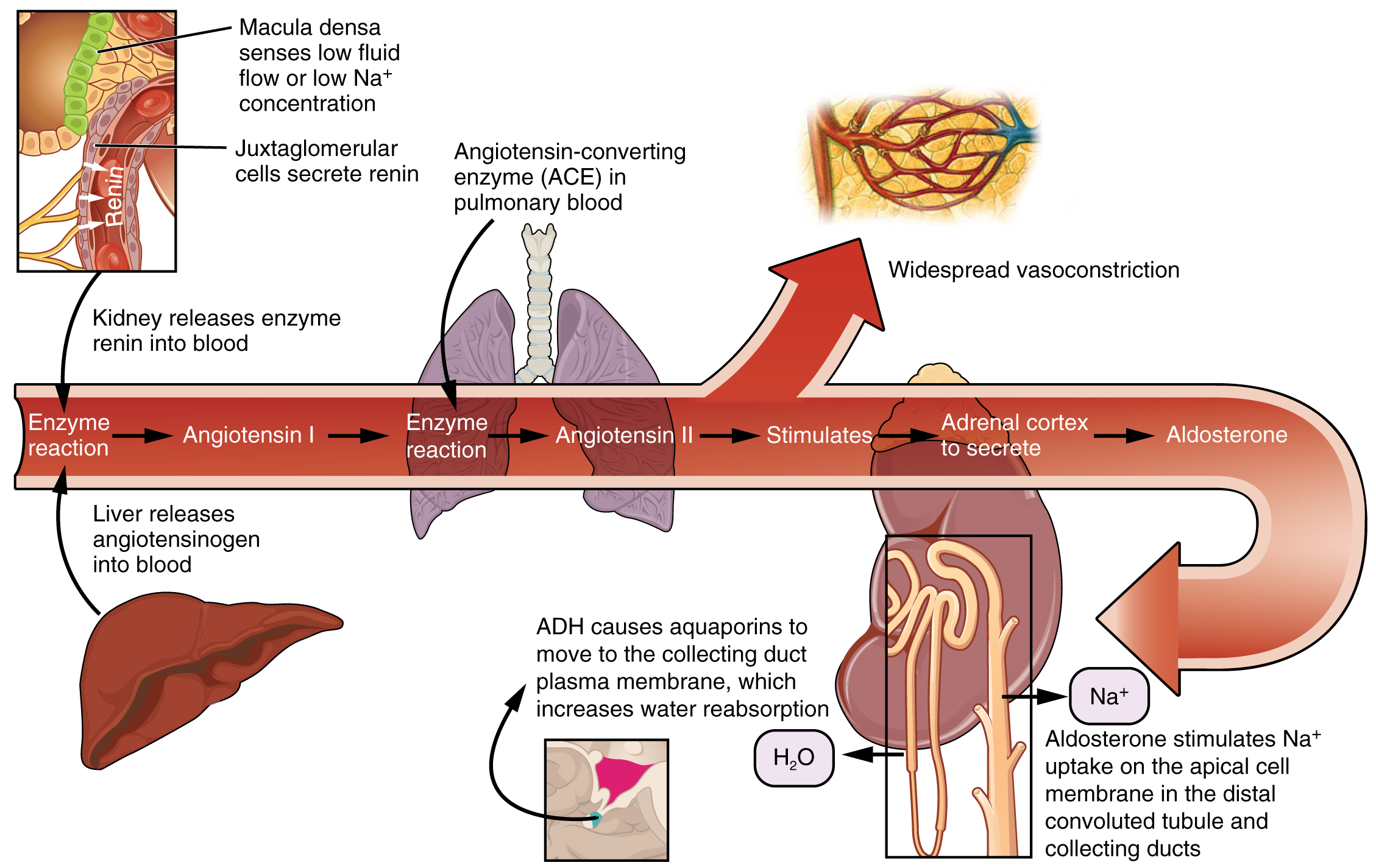
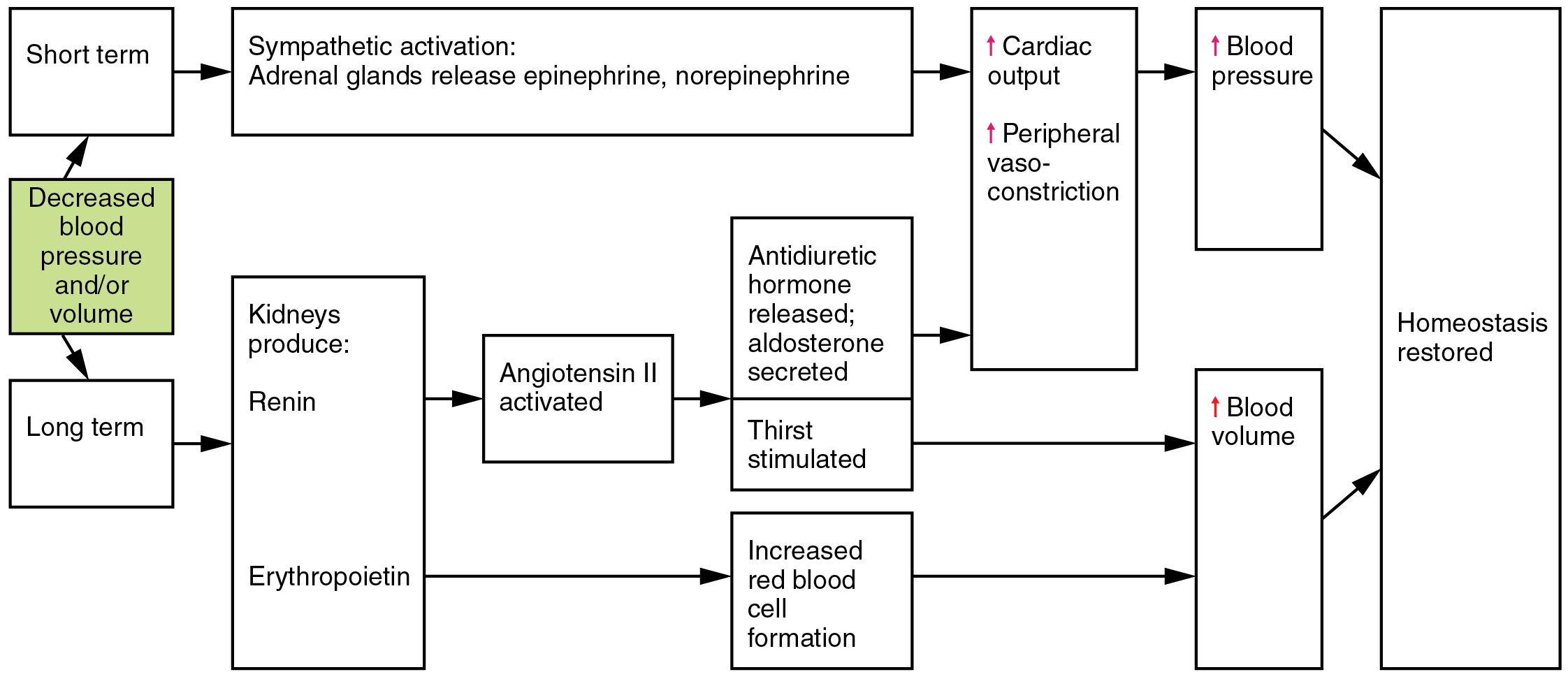
Autoregulation of Perfusion
Local, self-regulatory mechanisms allow each region of tissue to adjust its blood flow—and thus its perfusion. These mechanisms are affected by sympathetic and parasympathetic stimulation, as well as endocrine factors. See the following tables for a summary of these factors and their effects.[25]
| Factor | Vasoconstruction | Vasodilation |
|---|---|---|
| Sympathetic stimulation | Arterioles within integument abdominal viscera and mucosa membrane; skeletal muscles (at high levels); varied in veins and venules | Arterioles within heart; skeletal muscles at low to moderate levels |
| Parasympathetic | No known innervation for most | Arterioles in external genitalia; no known innervation for most other arterioles or veins |
| Factor | Vasoconstruction | Vasodilation |
|---|---|---|
| Epinephrine | Similar to sympathetic stimulation for extended fight-or-flight responses; at high levels, binds to specialized alpha (α) receptors | Similar to sympathetic stimulation for extended fight-or-flight responses; at low to moderate levels, binds to specialized beta (β) receptors |
| Norepinephrine | Similar to epinephrine | Similar to epinephrine |
| Angiotensin II | Powerful generalized vasoconstrictor; also stimulates release of aldosterone and ADH | n/a |
| ANH (peptide) | n/a | Powerful generalized vasodilator; also promotes loss of fluid volume from kidneys, hence reducing blood volume, pressure, and flow |
| ADH | Moderately strong generalized vasoconstrictor; also causes body to retain more fluid via kidneys, increasing blood volume and pressure | n/a |
| Factor | Vasoconstruction | Vasodilation |
|---|---|---|
| Decreasing levels of oxygen | n/a | Vasodilation, also opens precapillary sphincters |
| Decreasing pH | n/a | Vasodilation, also opens precapillary sphincters |
| Increasing levels of carbon dioxide | n/a | Vasodilation, also opens precapillary sphincters |
| Increasing levels of potassium ion | n/a | Vasodilation, also opens precapillary sphincters |
| Increasing levels of prostaglandins | Vasoconstriction, closes precapillary sphincters | Vasodilation, opens precapillary sphincters |
| Increasing levels of adenosine | n/a | Vasodilation |
| Increasing levels of lactic acid and other metabolites | n/a | Vasodilation, also opens precapillary sphincters |
| Increasing levels of endothelins | Vasoconstriction | n/a |
| Increasing levels of platelet secretions | Vasoconstriction | n/a |
| Increasing hypothermia | n/a | Vasodilation |
| Stretching of vascular wall (myogenic) | Vasoconstriction | n/a |
| Increasing levels of histamines from basophils and mast cells | n/a | Vasodilation |
Kidney Function Review
As discussed earlier, the kidney helps to regulate blood pressure, along with the heart and blood vessels, primarily through the Renin-Angiotensin-Aldosterone System (RAAS). In addition to cardiovascular medications affecting the RAAS system, there are also medications called diuretics that reduce blood volume by working at the nephron level. This section will review the basic concepts of kidney function at the nephron level to promote understanding of the mechanism of action of various cardiovascular medications.
The kidney receives blood from the circulatory system via the renal artery. The renal artery branches into smaller and smaller arterioles until the smallest arteriole, the afferent arteriole, services the nephrons. There are about 1.3 million nephrons in each kidney. Nephron’s role is to “clean” the blood from excessive wastes by extracting it out of the blood and forming it into urine by accomplishing three principal functions—filtration, reabsorption, and secretion. They also have additional secondary functions in regulating blood pressure (via the production of renin) and producing red blood cells (via the hormone erythropoietin).[26]
The initial filtering of the blood takes place in the glomerulus, a cluster of capillaries surrounded by the glomerular capsule. The rate at which this filtering occurs is called the glomerular filtration rate (GFR) and is used to gauge how well the kidneys are functioning. The rate at which blood flows into the glomerulus is controlled by afferent arterioles and the blood vessels flowing out of the glomerulus. These blood vessels are called called efferent arterioles.[27] See Figure 6.2m[28] for an illustration of blood flow through the kidney and nephrons.
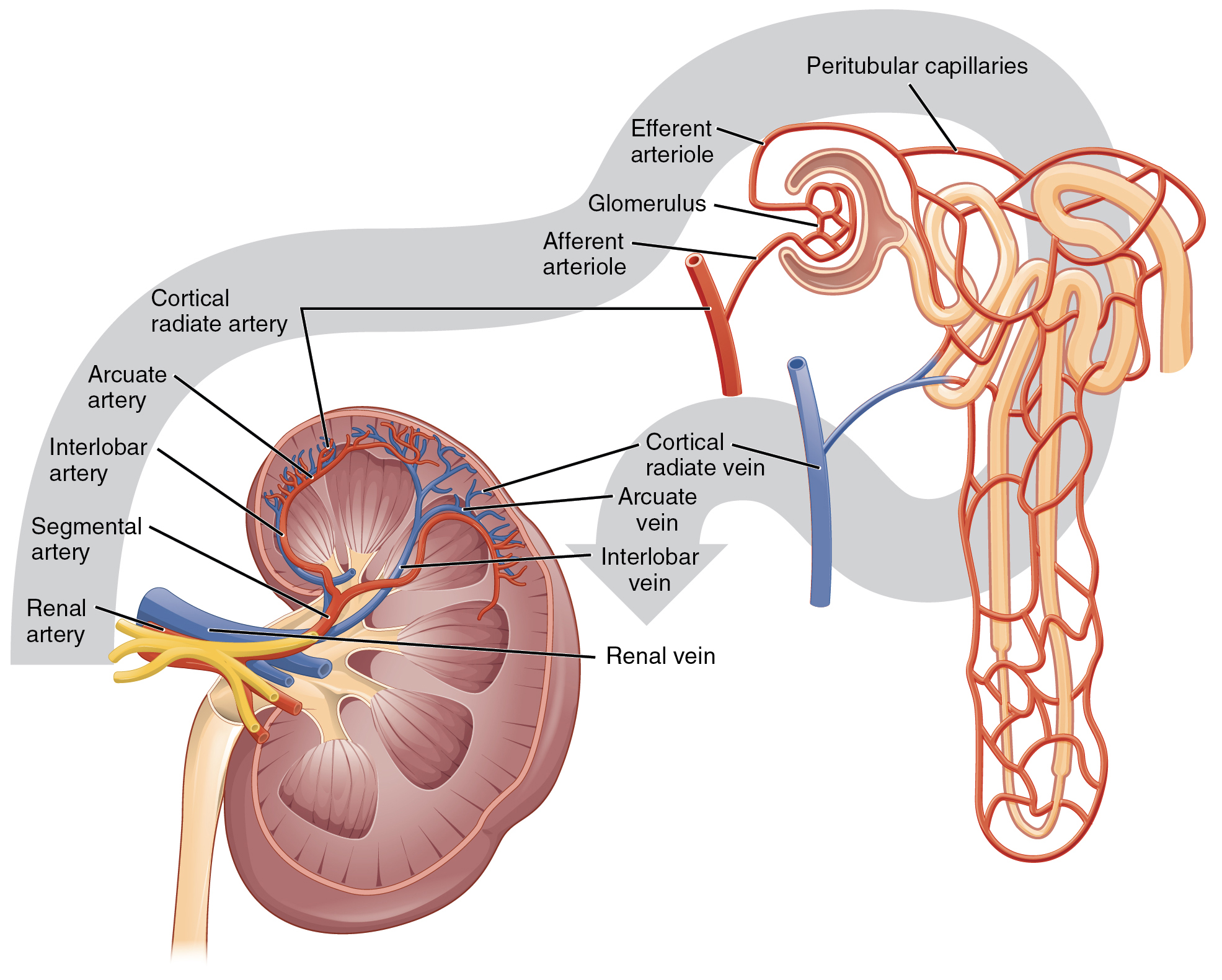
Lying just outside the glomerulus is the juxtaglomerular apparatus (JGA). One function of the JGA is to regulate renin release as part of the RAAS system discussed earlier in this chapter.
See Figure 6.2n[29] for an illustration of nephron structure. From the glomerulus (1), the proximal tubule (2) returns 60-70% of the sodium and water back into the bloodstream. From the proximal tubule, the filtrate flows into the descending Loop of Henle (3) and then the ascending Loop of Henle (4). Another 20-25% of sodium is reabsorbed in the ascending loop of Henle, and this is the site of action of loop diuretics. Filtrate then enters the distal tubule (5), where sodium is actively filtered in exchange for potassium or hydrogen ions, a process regulated by the hormone aldosterone. This is the site of action for thiazide diuretics. The collecting duct (6) is the final pathway; this is where antidiuretic hormone (ADH) acts to increase the absorption of water back into the bloodstream, thereby preventing it from being lost in the urine.[30]
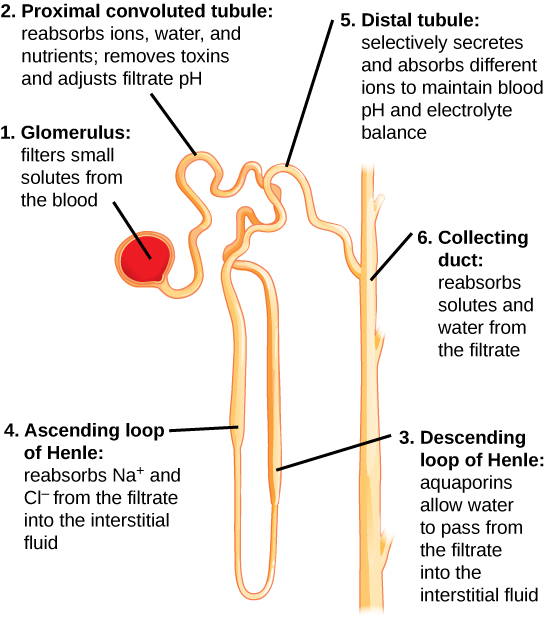
Elimination of Drugs and Hormones
Water-soluble drugs may be excreted in the urine and are influenced by one or all of the following processes: glomerular filtration, tubular secretion, or tubular reabsorption. Drugs that are structurally small can be filtered by the glomerulus with the filtrate. However, large drug molecules such as heparin or those that are bound to plasma proteins cannot be filtered and are not readily eliminated. Some drugs can be eliminated by carrier proteins that enable secretion of the drug into the tubule (such as dopamine or histamine).[31]
Blood and Coagulation
Now that we have reviewed the functions of the heart, blood vessels, and kidneys, we will review coagulation. As we discussed, the primary function of blood as it moves through the blood vessels in the body is to deliver oxygen and nutrients and remove wastes as it is filtered by the kidney, but that is only the beginning of the story. Cellular elements of blood include red blood cells (RBCs), white blood cells (WBCs), and platelets, and each element has its own function. Red blood cells carry oxygen; white blood cells assist with the immune response; and platelets are key players in hemostasis, the process by which the body seals a small ruptured blood vessel and prevents further loss of blood. There are three steps to the hemostasis process: vascular spasm, the formation of a platelet plug, and coagulation (blood clotting). Failure of any of these steps will result in hemorrhage (excessive bleeding). Each of these steps will be further discussed below.[32]
Vascular Spasm
When a vessel is severed or punctured or when the wall of a vessel is damaged, vascular spasm occurs. In vascular spasm, the smooth muscle in the walls of the vessel contracts dramatically. The vascular spasm response is believed to be triggered by several chemicals called endothelins that are released by vessel-lining cells and by pain receptors in response to vessel injury. This phenomenon typically lasts for up to 30 minutes, although it can last for hours.
Formation of the Platelet Plug
In the second step, platelets, which normally float free in the plasma, encounter the area of vessel rupture with the exposed underlying connective tissue and collagenous fibers. The platelets begin to clump together, become spiked and sticky, and bind to the exposed collagen and endothelial lining. This process is assisted by a glycoprotein in the blood plasma called von Willebrand factor, which helps stabilize the growing platelet plug. As platelets collect, they simultaneously release chemicals from their granules into the plasma that further contribute to hemostasis. Among the substances released by the platelets are:
- adenosine diphosphate (ADP), which helps additional platelets to adhere to the injury site, reinforcing and expanding the platelet plug
- serotonin, which maintains vasoconstriction
- prostaglandins and phospholipids, which also maintain vasoconstriction and help to activate further clotting chemicals
A platelet plug can temporarily seal a small opening in a blood vessel, thus buying the body more time while more sophisticated and durable repairs are being made.[33]
Coagulation
The more sophisticated and more durable repairs are called coagulation, or the formation of a blood clot. The process is sometimes characterized as a cascade because one event prompts the next as in a multi-level waterfall. The result is the production of a gelatinous but robust clot made up of a mesh of fibrin in which platelets and blood cells are trapped. Figure 6.2o[34] summarizes the three steps of hemostasis when an injury to a blood vessel occurs. First, vascular spasm constricts the flow of blood. Next, a platelet plug forms to temporarily seal small openings in the vessel. Coagulation then enables the repair of the vessel wall once the leakage of blood has stopped. The synthesis of fibrin in blood clots involves either an intrinsic pathway or an extrinsic pathway, both of which lead to a common pathway creating a clot.[35]
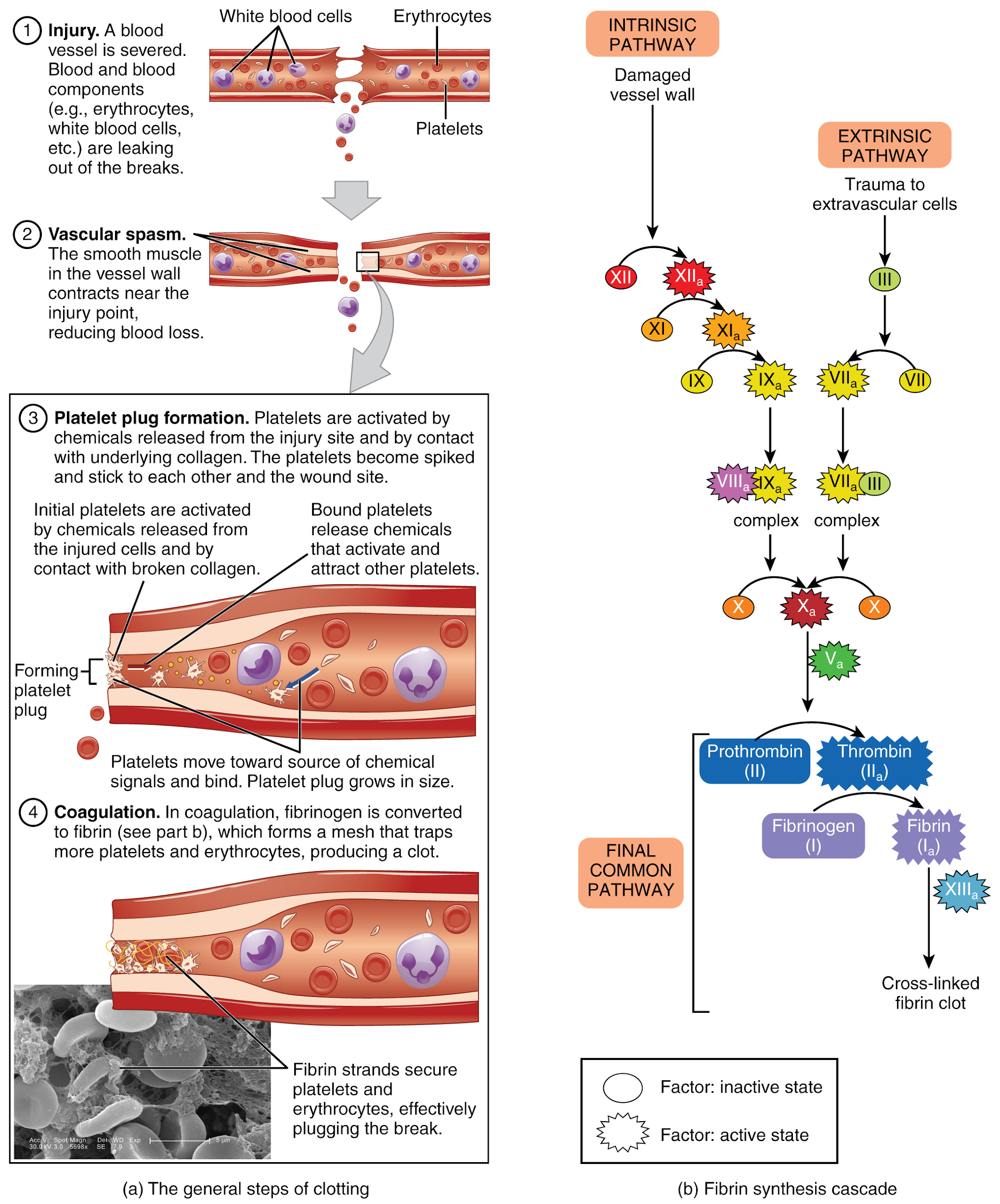
Extrinsic Pathway
The quicker responding and more direct extrinsic pathway (also known as the tissue factor pathway) begins when damage occurs to the surrounding tissues, such as in a traumatic injury. The events in the extrinsic pathway are completed in a matter of seconds.
Intrinsic Pathway
The intrinsic pathway is longer and more complex. In this case, the factors involved are intrinsic to (present within) the bloodstream. The pathway can be prompted by damage to the tissues or resulting from internal factors such as arterial disease. The events in the intrinsic pathway are completed in a few minutes.
Common Pathway
Both the intrinsic and extrinsic pathways lead to the common pathway, where fibrin is produced to seal off the vessel. Once Factor X has been activated by either the intrinsic or extrinsic pathway, Factor II, the inactive enzyme prothrombin, is converted into the active enzyme thrombin. Then thrombin converts Factor I, the soluble fibrinogen, into the insoluble fibrin protein strands. Factor XIII then stabilizes the fibrin clot.
Fibrinolysis
The stabilized clot is acted on by contractile proteins within the platelets. As these proteins contract, they pull on the fibrin threads, bringing the edges of the clot more tightly together, somewhat as we do when tightening loose shoelaces. This process also wrings out of the clot a small amount of fluid called serum, which is blood plasma without its clotting factors.
To restore normal blood flow as the vessel heals, the clot must eventually be removed. Fibrinolysis is the gradual degradation of the clot. Again, there is a fairly complicated series of reactions that involves Factor XII and protein-catabolizing enzymes. During this process, the inactive protein plasminogen is converted into the active plasmin, which gradually breaks down the fibrin of the clot. Additionally, bradykinin, a vasodilator, is released, reversing the effects of the serotonin and prostaglandins from the platelets. This allows the smooth muscle in the walls of the vessels to relax and helps to restore the circulation.
Plasma Anticoagulants
An anticoagulant is any substance that opposes coagulation. Several circulating plasma anticoagulants play a role in limiting the coagulation process to the region of injury and restoring a normal, clot-free condition of blood. For instance, antithrombin inactivates Factor X and opposes the conversion of prothrombin (Factor II) to thrombin in the common pathway. Basophils release heparin, a short-acting anticoagulant that also opposes prothrombin. A pharmaceutical form of heparin is often administered therapeutically to prevent or treat blood clots.
A thrombus is an aggregation of platelets, erythrocytes, and even WBCs typically trapped within a mass of fibrin strands. While the formation of a clot is normal following the hemostatic mechanism just described, thrombi can form within an intact or only slightly damaged blood vessel. In a large vessel, a thrombus will adhere to the vessel wall and decrease the flow of blood. In a small vessel, it may actually totally block the flow of blood and is termed an occlusive thrombus.
There are several medications that impact the coagulation cascade. For example, aspirin (acetylsalicylic acid) is very effective at inhibiting the aggregation of platelets. Clients at risk for cardiovascular disease often take a low dose of aspirin on a daily basis as a preventive measure. It is also routinely administered during a heart attack or stroke to reduce the formation of the platelet plug. Anticoagulant medications such as warfarin and heparin prevent the formation of clots by affecting the intrinsic or extrinsic pathways. Another class of drugs that are known as thrombolytic agents is used to dissolve an abnormal clot. If a thrombolytic agent is administered to a client within a few hours following a thrombotic stroke or myocardial infarction, the client’s prognosis improves significantly. Tissue plasminogen activator (TPA) is an example of a medication that is released naturally by endothelial cells but is also used in clinical medicine to break down a clot.[36]
Video Review of Basic Concepts
For additional video review of the basic anatomy and physiology concepts of the cardiovascular and renal system, see the supplementary videos below.[37]
Blood Vessels
Muscle Contraction[38]
Fluids and Electrolytes: Potassium and Aldosterone[39]
Fluid and Electrolytes: Sodium[40]
Anatomy of the Heart[41]
The Blood[42]
Anatomy of Urinary System[43]
Renin-Angiotensin System[44]
Introduction to ECG[45]
Circulatory System Anatomy[46]
Image Description
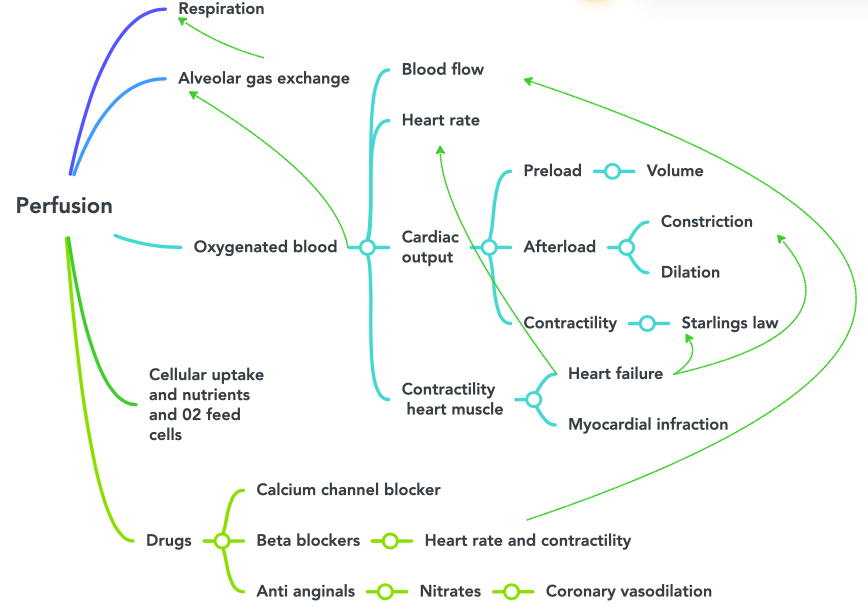
Figure 6.2a Perfusion concept map image description:
This concept map illustrates the steps of perfusion. The flow is as follows:
Respiration
- Alveolar gas exchange
- Oxygenated blood
- Blood flow
- Heart rate
- Cardiac output (this also connects to alveolar gas exchange)
- Preload
- Volume
- Afterload
- Constriction
- Dilation
- Contractility
- Starlings law
- Preload
- Contractility heart muscle
- Heart failure (this also connects to heart rate, Starlings law and constriction)
- Myocardial infraction
- Cellular uptake and nutrientss and 02 feed cells
- Drugs
- Calcium channel blocker
- Beta blockers
- Heart rate and contractility (this also connects to blood flow)
- Anti anginals
- Nitrates
- Coronary vasodilation
- Nitrates
Figure 6.2g Factors affecting cardiac output
Factors affecting heart rate (HR):
- autonomic innervation
- hormones
- fitness levels
- age
Factors affecting stroke volume (SV):
- heart size
- fitness levels
- gender
- contractility
- duration of contraction
- preload (EDV)
- afterload (resistance)
- Stroke Volume (SV) = EDV − ESV
Figure 6.2i Factors affecting cardiac output
Given the formula: Cardiac Output (CO) = HR × SV, Stroke Volume (SV) = EDV − ESV
Factors affecting heart rate (HR):
- atrial reflex
- autonomic innervation
- hormones
Factors affecting stroke volume (SV):
- preload
- affected by venous return and filling time
- affects end diastolic volume (EDV) and end systolic volume (ESV)
- contractility
- affected by autonomic innervation and hormones
- affects end systolic volume (ESV)
- afterload
- affected by vasodilation or vasoconstriction
- affects end systolic volume (ESV)
Figure 6.2k The renin-angiotensin-aldosterone system
- Enzyme reaction
- Macula densa senses low fluid flow or low Na+ concentration
- Juxtaglomerular cells secrete renin
- Kidney releases enzyme renin into blood
- Liver releases angiotensinogen into blood
- Angiotensin I
- Enzyme reaction
- Angiotensin-converting enzyme (ACE) in pulmonary blood
- Angiotensin II
- Widespread vasoconstriction
- Stimulates
- Adrenal cortex to secrete
- Aldosterone
- Aldosterone stimulates Na+ uptake on the apical cell membrane in the distal convoluted tubule and collecting ducts
- H2O
- ADH causes aquaporins to move to the collecting duct plasma membrane, which increases water reabsorption
Figure 6.2l Hormones involved in renal control of blood pressure
Decreased blood pressure and/or volume in short term:
- Sympathetic activation: adrenal glands release epinephrine, norepinephrine
- Increases cardiac output and peripheral vaso-constriction
- Increases blood pressure
- Homeostasis restored
Decreased blood pressure and/or volume in long term:
- Kidneys produces:
- Renin
- Angiotensin II activated
- Antidiuretic hormone released; aldosterone secreted. Thirst stimulated.
- Erythropoietin
- Increased red blood cell formation
- Renin
- Increases blood pressure
- Homeostasis restored
- "Position of the Heart in the Thorax" by OpenStax College is licensed under CC BY 4.0. Access for free at https://openstax.org/books/anatomy-and-physiology/pages/19-1-heart-anatomy ↵
- This work is a derivative of Anatomy and Physiology by OpenStax licensed under CC BY 4.0. Access for free at https://openstax.org/books/anatomy-and-physiology/pages/1-introduction ↵
- "Dual System of the Human Blood Circulation" by OpenStax College is licensed under CC By 4.0. Access for free at https://openstax.org/books/anatomy-and-physiology/pages/19-1-heart-anatomy ↵
- This work is a derivative of Anatomy and Physiology by OpenStax licensed under CC BY 4.0. Access for free at https://openstax.org/books/anatomy-and-physiology/pages/1-introduction. ↵
- "Surface Anatomy of the Heart" by OpenStax College is licensed under CC BY 4.0 Access for free at https://openstax.org/books/anatomy-and-physiology/pages/19-1-heart-anatomy ↵
- "2018 Conduction System of the Heart" by OpenStax College is licensed under CC BY 4.0 Access it for free at https://openstax.org/books/anatomy-and-physiology/pages/19-2-cardiac-muscle-and-electrical-activity ↵
- This work is a derivative of Anatomy and Physiology by OpenStax licensed under CC BY 4.0. Access for free at https://openstax.org/books/anatomy-and-physiology/pages/1-introduction ↵
- This work is a derivative of Anatomy and Physiology by OpenStax licensed under CC BY 4.0. Access for free at https://openstax.org/books/anatomy-and-physiology/pages/1-introduction ↵
- "Electrocardiogram Depolarization.jpg" by OpenStax College is licensed under CC BY 4.0. Access for free at https://openstax.org/books/anatomy-and-physiology/pages/19-2-cardiac-muscle-and-electrical-activity ↵
- This work is a derivative of Anatomy and Physiology by OpenStax licensed under CC BY 4.0. Access for free at https://openstax.org/books/anatomy-and-physiology/pages/1-introduction ↵
- This work is a derivative of Anatomy and Physiology by OpenStax licensed under CC BY 4.0. Access for free at https://openstax.org/books/anatomy-and-physiology/pages/1-introduction ↵
- "2031 Factors in Cardiac Output.jpg" by OpenStax College is licensed under CC BY 4.0 Access for free at https://openstax.org/books/anatomy-and-physiology/pages/19-4-cardiac-physiology ↵
- This work is a derivative of Anatomy and Physiology by OpenStax licensed under CC BY 4.0. Access for free at https://openstax.org/books/anatomy-and-physiology/pages/1-introduction ↵
- "2032 Automatic Innervation.jpg" by OpenStax College is licensed under CC BY 4.0 Access for free at https://openstax.org/books/anatomy-and-physiology/pages/19-4-cardiac-physiology ↵
- This work is a derivative of Anatomy and Physiology by OpenStax licensed under CC BY 4.0. Access for free at https://openstax.org/books/anatomy-and-physiology/pages/1-introduction ↵
- This work is a derivative of Anatomy and Physiology by OpenStax licensed under CC BY 4.0. Access for free at https://openstax.org/books/anatomy-and-physiology/pages/1-introduction ↵
- "2036 Summary of Factors in Cardiac Output.jpg" by OpenStax College is licensed under CC BY 4.0 Access for free at https://openstax.org/books/anatomy-and-physiology/pages/19-4-cardiac-physiology ↵
- This work is a derivative of Anatomy and Physiology by OpenStax licensed under CC BY 4.0. Access for free at https://openstax.org/books/anatomy-and-physiology/pages/1-introduction ↵
- This work is a derivative of Anatomy and Physiology by OpenStax licensed under CC BY 4.0. Access for free at https://openstax.org/books/anatomy-and-physiology/pages/1-introduction ↵
- "Combinpedal.jpg" by James Heilman, MD is licensed under CC BY-SA 3.0 ↵
- This work is a derivative of Anatomy and Physiology by OpenStax licensed under CC BY 4.0. Access for free at https://openstax.org/books/anatomy-and-physiology/pages/1-introduction ↵
- This work is a derivative of Anatomy and Physiology by OpenStax licensed under CC BY 4.0. Access for free at https://openstax.org/books/anatomy-and-physiology/pages/1-introduction ↵
- "2626 Renin Aldosterone Angiotensin.jpg" by OpenStax College is licensed under CC BY 4.0 Access for free at https://openstax.org/books/anatomy-and-physiology/pages/25-4-microscopic-anatomy-of-the-kidney ↵
- This work is a derivative of Anatomy and Physiology by OpenStax licensed under CC BY 4.0. Access for free at https://openstax.org/books/anatomy-and-physiology/pages/1-introduction ↵
- This work is a derivative of Anatomy and Physiology by OpenStax licensed under CC BY 4.0. Access for free at https://openstax.org/books/anatomy-and-physiology/pages/1-introduction ↵
- This work is a derivative of Anatomy and Physiology by OpenStax licensed under CC BY 4.0. Access for free at https://openstax.org/books/anatomy-and-physiology/pages/1-introduction. ↵
- McCuistion, L., Vuljoin-DiMaggio, K., Winton, M, & Yeager, J. (2018). Pharmacology: A patient-centered nursing process approach. pp. 443-454. Elsevier. ↵
- "2612 Blood Flow in the Kidneys.jpg" by OpenStax College is licensed under CC BY 4.0 Access for free at https://openstax.org/books/anatomy-and-physiology/pages/25-3-gross-anatomy-of-the-kidney ↵
- "Figure 41 03 04.jpg" by CNX OpenStax is licensed under CC BY 4.0 ↵
- McCuistion, L., Vuljoin-DiMaggio, K., Winton, M, & Yeager, J. (2018). Pharmacology: A patient-centered nursing process approach. pp. 443-454. Elsevier. ↵
- This work is a derivative of Anatomy and Physiology by OpenStax licensed under CC BY 4.0. Access for free at https://openstax.org/books/anatomy-and-physiology/pages/1-introduction ↵
- This work is a derivative of Anatomy and Physiology by OpenStax licensed under CC BY 4.0. Access for free at https://openstax.org/books/anatomy-and-physiology/pages/1-introduction ↵
- This work is a derivative of Anatomy and Physiology by OpenStax licensed under CC BY 4.0. Access for free at https://openstax.org/books/anatomy-and-physiology/pages/1-introduction ↵
- "1909 Blood Clotting.jpg" by OpenStax College is licensed under CC BY 4.0 Access for free at https://openstax.org/books/anatomy-and-physiology/pages/18-5-hemostasis ↵
- This work is a derivative of Anatomy and Physiology by OpenStax licensed under CC BY 4.0. Access for free at https://openstax.org/books/anatomy-and-physiology/pages/1-introduction ↵
- This work is a derivative of Anatomy and Physiology by OpenStax licensed under CC BY 4.0. Access for free at https://openstax.org/books/anatomy-and-physiology/pages/1-introduction ↵
- Forciea, B. (2018, April 26). Structure of Arteries and Veins V2. [Video]. YouTube. All rights reserved. Video used with permission. https://youtu.be/HZAeua5JbrU ↵
-
Forciea, B. (2016, September 14). Muscle Contraction Physiology. [Video]. YouTube. All rights reserved. Video used with permission. https://youtu.be/TB7TypeksGk ↵
- Forciea, B. (2017, April 26). Fluids and Electrolytes Potassium. [Video]. YouTube. All rights reserved. Video used with permission. https://youtu.be/SNAiGaaYkvs ↵
- Forciea, B. (2017, April 24). Fluids and Electrolytes Sodium. [Video]. YouTube. All rights reserved. Video used with permission. https://youtu.be/ar-WrfC7SJs ↵
- Forciea, B. (2015, May 20). Anatomy of the Heart (v2.0). [Video]. YouTube. All rights reserved. Video used with permission. https://youtu.be/d8RSvcc8koo ↵
- Forciea, B. (2015, May 19). Anatomy and Physiology: The Blood. [Video]. YouTube. All rights reserved. Video used with permission. https://youtu.be/bjfcOSoDSzg ↵
- Forciea, B. (2015, May 13). Urinary System Anatomy (v2.0) [Video]. YouTube. All rights reserved. Video used with permission. https://youtu.be/2Wd45Zmq_Ck ↵
- Forciea, B. (2015, May 13). Renin-Angiotensin System for Anatomy and Physiology (v2.0) [Video]. YouTube. All rights reserved. Video used with permission. https://youtu.be/iin4lbAKv7Q ↵
- Forciea, B. (2015, May 12). Introduction to the Electrocardiogram (ECG) V2.0. [Video]. YouTube. All rights reserved. Video used with permission. https://youtu.be/mAN0GK7O9yU ↵
- Forciea, B. (2015, May 12). Circulatory System Anatomy (v2.0). [Video]. YouTube. All rights reserved. Video used with permission. https://youtu.be/nBSHhkOEKHA ↵
Normal cardiac rhythm is established by the sinoatrial (SA) node. The SA node has the highest inherent rate of depolarization and is known as the pacemaker of the heart.
Normal electrical pattern followed by contraction of the heart.
The period of contraction that the heart undergoes while it pumps blood into circulation.
The period of relaxation that occurs as the chambers of the heart fill with blood.
The amount of blood in the atria just prior to atrial contraction.
The amount of blood that both ventricles pump during each contraction, normally in the range of 70–80 mL.
A measurement of the amount of blood pumped by each ventricle in one minute.To calculate this value, multiply stroke volume (SV), the amount of blood pumped by each ventricle, by heart rate (HR), in contractions per minute (or beats per minute, bpm). It can be represented mathematically by the following equation: CO = HR × SV.
The force of contraction of the heart.
The tension that the ventricles must develop to pump blood effectively against the resistance in the vascular system.
Factors that increase contractility.
Factors that decrease contractility.
A blood vessel that carries blood away from the heart (except for pulmonary arteries that carry oxygenated blood from the lungs back to the heart).
Smallest arteries where nutrients and wastes are exchanged at the cellular level.
Blood vessels that conduct blood toward the heart (except for pulmonary veins that carry deoxygenated blood from the heart to the lungs).
Volume of blood located in venous networks within the liver, bone marrow, and integument.
The presence of excess tissue fluid around the cells.
A type of hydrostatic pressure, or the force exerted by blood on the walls of the blood vessels or the chambers of the heart.
The ability of any compartment to expand to accommodate increased content. The greater the compliance of an artery, the more effectively it is able to expand to accommodate surges in blood flow without increased resistance or blood pressure. Veins are more compliant than arteries and can expand to hold more blood. When vascular disease causes stiffening of arteries, compliance is reduced and resistance to blood flow is increased.
A condition when compliance in an artery is reduced and pressure and resistance within the vessel increase. This is a leading cause of hypertension and coronary heart disease, as it causes the heart to work harder to generate a pressure great enough to overcome the resistance.
Decreased blood volume that may be caused by bleeding, dehydration, vomiting, severe burns, or by diuretics used to treat hypertension. Treatment typically includes intravenous fluid replacement.
Excessive fluid volume caused by retention of water and sodium, as seen in patients with heart failure, liver cirrhosis, and some forms of kidney disease.
Specialized cells in the kidneys that respond to decreased blood flow by secreting renin into the blood. Renin converts the plasma protein angiotensinogen into its active form—angiotensin I. Angiotensin I circulates in the blood and is then converted into angiotensin II in the lungs. This reaction is catalyzed by the enzyme angiotensin-converting enzyme (ACE). Angiotensin II is a powerful vasoconstrictor, greatly increasing blood pressure. It also stimulates the release of ADH and aldosterone, a hormone produced by the adrenal cortex. Aldosterone increases the reabsorption of sodium into the blood by the kidneys causing reabsorption of water and increasing blood volume and raising blood pressure.
A component of the nephron where loop diuretics act to eliminate sodium and water
The process by which the body temporarily seals a ruptured blood vessel and prevents further loss of blood.
The formation of a blood clot.
The gradual degradation of a clot.
An aggregation of platelets, erythrocytes, and WBCs trapped within a mass of fibrin strands that adhere to the vessel wall and decrease the flow of blood or totally block the flow of blood.

