Adrenal: A&P Basics Review
The adrenal gland consists of the adrenal cortex that is composed of glandular tissue and the adrenal medulla that is composed of nervous tissue. Each region secretes its own set of hormones.
The adrenal cortex is a component of the hypothalamic-pituitary-adrenal (HPA) axis. The hypothalamus stimulates the release of ACTH from the pituitary, which then stimulates the adrenal cortex to produce steroid hormones that are important for the regulation of the stress response, blood pressure and blood volume, nutrient uptake and storage, fluid and electrolyte balance, and inflammation.
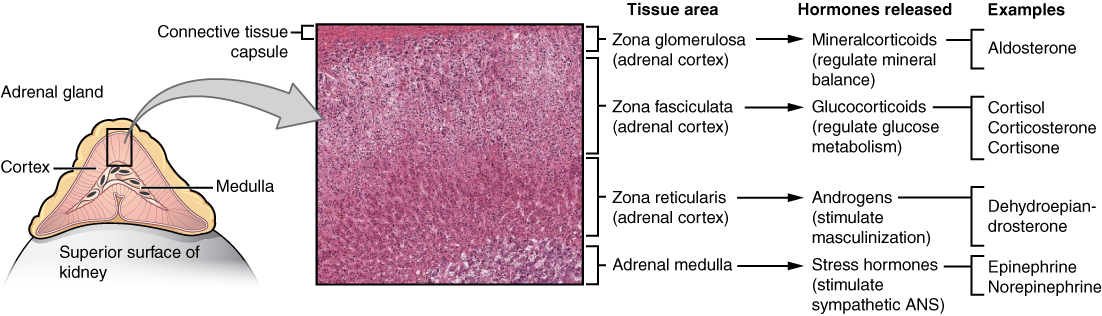
The adrenal medulla is neuroendocrine tissue composed of postganglionic sympathetic nervous system (SNS) neurons, that secretes the hormones epinephrine and norepinephrine. It is an extension of the autonomic nervous system, which regulates homeostasis in the body. See Figure 9.4a for an illustration of the adrenal gland and associated hormones.[1]
One of the major functions of the adrenal gland is to respond to stress. The body responds in different ways to short-term stress and long-term stress, following a pattern known as the general adaptation syndrome (GAS). Stage one of GAS is called the alarm reaction. This is short-term stress, also called the fight-or-flight response, and is mediated by the hormones epinephrine and norepinephrine from the adrenal medulla. Their function is to prepare the body for extreme physical exertion. If the stress is not soon relieved, the body adapts to the stress in the second stage called the stage of resistance. If a person is starving for example, the body may send signals to the gastrointestinal tract to maximize the absorption of nutrients from food. If the stress continues for a longer term however, the body responds with symptoms such as depression, suppressed immune response, or severe fatigue. These symptoms are mediated by the hormones of the adrenal cortex, especially cortisol.Adrenal hormones also have several non–stress-related functions, including the increase of blood sodium and glucose levels, which will be described in further detail below.
Mineralocorticoids: Aldosterone
The most superficial region of the adrenal cortex is the zona glomerulosa, which produces a group of hormones collectively referred to as mineralocorticoids because of their effect on body minerals, especially sodium and potassium. These hormones are essential for fluid and electrolyte balance.
Aldosterone is the major mineralocorticoid that is important in the regulation of the concentration of sodium and potassium ions in the body. The secretion of aldosterone by the adrenal cortex is prompted by the HPA axis when the hypothalamus triggers ACTH release from the anterior pituitary. It is released in response to elevated blood levels of potassium (K+), low blood levels of sodium (Na+), low blood pressure, or low blood volume. Aldosterone targets the kidneys and increases the excretion of K+ and the retention of Na+, which, in turn, causes the retention of water, thus increasing blood volume and blood pressure.
Aldosterone is also a key component of the renin-angiotensin-aldosterone system (RAAS) in which specialized cells of the kidneys secrete renin in response to low blood volume or low blood pressure. Renin then catalyzes the conversion of the blood protein angiotensinogen, which is produced by the liver, to the hormone Angiotensin I. Angiotensin I is converted in the lungs to Angiotensin II by the angiotensin-converting enzyme (ACE). Angiotensin II has three major functions: initiating vasoconstriction of the arterioles, thus decreasing blood flow; stimulating kidney tubules to reabsorb sodium and water, thus increasing blood volume; and signaling the adrenal cortex to secrete aldosterone, which further increases blood volume and blood pressure. It is important to understand these effects because many cardiac medications target the effects of aldosterone and the RAAS system. For example, drugs that block the production of Angiotensin II are known as ACE inhibitors. ACE inhibitors are used to help lower blood pressure in clients with hypertension by blocking the ACE enzyme from converting Angiotensin I to Angiotensin II, which, in turn, causes vasodilation of the arterioles. Another medication called spironolactone is used as a diuretic because it blocks the effects of aldosterone and, thus, causes the kidneys to eliminate water and sodium to decrease blood volume and blood pressure.
Glucocorticoids: Cortisol
The intermediate region of the adrenal cortex produces hormones called glucocorticoids because of their role in glucose metabolism. In response to long-term stressors, the HPA axis triggers the release of glucocorticoids. Their overall effect is to inhibit tissue building while stimulating the breakdown of stored nutrients to maintain adequate fuel supplies. In conditions of long-term stress, cortisol promotes the catabolism of glycogen to glucose, stored triglycerides into fatty acids and glycerol, and muscle proteins into amino acids. These raw materials can then be used to synthesize additional glucose and ketones for use as body fuels. However, the negative effects of catabolism for energy can result in muscle breakdown and weakness, poor wound healing, and the suppression of the immune system.
Many medications contain glucocorticoids to treat various conditions, such as cortisone injections for inflamed joints; prednisone tablets, IV medication, and steroid-based inhalers to manage inflammation that occurs in asthma; and hydrocortisone creams that are applied to relieve itchy skin rashes.
Androgens
The deepest region of the adrenal cortex produces small amounts of a class of steroid sex hormones called androgens. During puberty and most of adulthood, androgens are produced in the gonads. The androgens produced in the adrenal cortex supplement the gonadal androgens.
Adrenal Medulla: Epinephrine and Norepinephrine
As noted earlier, the adrenal cortex releases glucocorticoids in response to long-term stress such as severe illness. In contrast, the adrenal medulla releases its hormones in response to acute, short-term stress mediated by the sympathetic nervous system (SNS). The medullary tissue is composed of unique postganglionic SNS neurons called chromaffin cells that produce the neurotransmitters epinephrine (also called adrenaline) and norepinephrine (also called noradrenaline), which are chemically classified as catecholamines. Epinephrine is produced in greater quantities and is the more powerful hormone.
The secretion of medullary epinephrine and norepinephrine is controlled by a neural pathway that originates from the hypothalamus in response to danger or stress. Both epinephrine and norepinephrine increase the heart rate, pulse, and blood pressure to prepare the body to fight the perceived threat or flee from it. In addition, the pathway dilates the airways, raising blood oxygen levels. It also prompts vasodilation, further increasing the oxygenation of important organs such as the lungs, brain, heart, and skeletal muscle while also prompting vasoconstriction to blood vessels serving less essential organs such as the gastrointestinal tract, kidneys, and skin. It also downregulates some components of the immune system. Other effects include a dry mouth, loss of appetite, pupil dilation, and a loss of peripheral vision.
Disorders Involving the Adrenal Glands
Several disorders are caused by the dysregulation of the hormones produced by the adrenal glands. For example, Cushing’s disease is a disorder characterized by high blood glucose levels, the development of a moon-shaped face, a buffalo hump on the back of the neck, rapid weight gain, and hair loss. It is caused by hypersecretion of cortisol. Cushing’s syndrome can also be caused by long-term use of corticosteroid medications.
In contrast, the hyposecretion of corticosteroids can result in Addison’s disease, a disorder that causes low blood glucose levels and low blood sodium levels. Addisonian crisis is a life-threatening condition due to severely low blood pressure resulting from a lack of corticosteroid levels.[2],[3],[4],[5]
A supplementary video about ACTH and the adrenal gland is provided below.
ACTH and the Adrenal Gland[6]
Nursing Considerations for Adrenal Medications
Assessment
Before initiating long-term systemic corticosteroid therapy, a thorough history and physical examination should be performed to assess for risk factors or pre-existing conditions that may potentially be exacerbated by glucocorticoid therapy, such as diabetes, dyslipidemia, cerebrovascular disease (CVD), GI disorders, affective disorders, or osteoporosis. At a minimum, baseline measures of body weight, height, bone mineral density, and blood pressure should be obtained, along with laboratory assessments that include a complete blood count (CBC), blood glucose values, and lipid profile. In children, nutritional and pubertal status should also be examined. Symptoms of and/or exposure to serious infections should also be assessed as corticosteroids are contraindicated in clients with untreated systemic infections. Concomitant use of other medications should also be assessed before initiating therapy as significant drug interactions have been noted between glucocorticoids and several drug classes. Females of childbearing age should also be questioned about the possibility of pregnancy because use in pregnancy may increase the risk of cleft palate in offspring.[7]
Implementation
Long-term corticosteroid therapy should never be stopped abruptly due to its effect on the hypothalamic-pituitary-adrenal (HPA) axis and potential adrenal suppression. Instead, the dose should be tapered to allow the body to resume natural production of adrenal hormone levels.
Clients on long-term corticosteroid therapy who are also at high risk for fractures are recommended to receive concurrent pharmacological treatment for osteoporosis. Alendronate, a bisphosphonates class of medication, is often used in addition to other osteoporosis preventative measures such as weight-bearing exercise and calcium/Vitamin D supplementation.[8]
Evaluation
The lowest effective dose should be used for treatment of the underlying condition, and the dose should be re-evaluated regularly to determine if further reductions can be instituted.
The parameters described under “Assessment” should be monitored regularly. Health care professionals should monitor for adrenal suppression in clients who have been treated with corticosteroids for greater than two weeks or in multiple short courses of high-dose therapy. Symptoms of adrenal insufficiency include weakness/fatigue, malaise, nausea, vomiting, diarrhea, abdominal pain, headache (usually in the morning), poor weight gain and/or growth in children, myalgia, arthralgia, psychiatric symptoms, hypotension, and hypoglycemia. If these symptoms occur, further lab work, such as an early morning cortisol test, should be performed.[9]
Adrenal Medication: Corticosteroids
Indications
Corticosteroids are used as replacement therapy in adrenal insufficiency, as well as for the management of various dermatologic, ophthalmologic, rheumatologic, pulmonary, hematologic, and gastrointestinal (GI) disorders. In respiratory conditions, systemic corticosteroids are used for the treatment of acute exacerbations of chronic obstructive pulmonary disease (COPD) and severe asthma. Mineralocorticoids are primarily involved in the regulation of electrolyte and water balance. Glucocorticoids are predominantly involved in carbohydrate, fat, and protein metabolism and also have anti-inflammatory, immunosuppressive, anti-proliferative, and vasoconstrictive effects. Prednisone is perhaps the most widely used of the systemic corticosteroids. It is generally used as an anti-inflammatory and immunosuppressive agent. Hydrocortisone is a commonly used topical cream for itching, and its oral formulation is used to treat Addison’s disease.[10] Methylprednisolone is a commonly used injectable corticosteroid. Fludrocortisone has much greater mineralocorticoid potency and, therefore, is commonly used to replace aldosterone in Addison’s disease.[11] See Figure 9.4b-d for images of various formulations of corticosteroids.[12],[13],[14]

Corticosteroids are used for a variety of disorders such as:
- Endocrine disorders such as adrenocortical insufficiency
- Rheumatic disorders such as rheumatoid arthritis
- Collagen diseases such as systemic lupus erythematosus
- Dermatologic diseases such as severe psoriasis
- Allergic states such as contact dermatitis or drug hypersensitivity reactions
- Ophthalmic diseases such as optic neuritis
- Respiratory diseases such as asthma or COPD
- Neoplastic diseases such as leukemia
- Gastrointestinal diseases such as ulcerative colitis
- Nervous system diseases such as multiple sclerosis [15]
Mechanism of Action
Glucocorticoids cause profound and varied metabolic effects as described in the “Adrenal A&P Basics Review” section above. In addition, they modify the body’s immune responses.[16]
Specific Administration Considerations
Despite their beneficial effects, long-term systemic use of corticosteroids is associated with well-known adverse events, including osteoporosis and fractures, adrenal suppression, hyperglycemia and diabetes, cardiovascular disease and dyslipidemia, dermatological and GI events, psychiatric disturbances, and immunosuppression. One side effect that is unique to children is growth suppression.[17] Therefore, the lowest possible dose of corticosteroid should be used to control the condition under treatment to avoid the development of these adverse effects. When reduction in dosage is possible, the reduction should be gradual and should not be stopped abruptly because of the associated HPA suppression that occurs with long-term administration. This hypothalamus-pituitary-adrenal (HPA) suppression can cause an impaired stress response, which may persist for months after discontinuation of therapy; therefore, in any situation of stress occurring during that period, hormone therapy should be reinstituted. Alternate day therapy is a corticosteroid dosing regimen in which twice the usual daily dose of corticoid is administered every other morning. The purpose of this mode of therapy is to minimize undesirable effects that can occur during long-term administration.
Dosages are variable and tailored to the disease process and the individual.
Adverse/side effects
Adverse/side effects of corticosteroids include fluid and electrolyte imbalances; muscle weakness; peptic ulcers; thin, fragile skin that bruises easily; poor wound healing; and the development of Cushing’s syndrome. Corticosteroids may mask some signs of infection, and new infections may appear during their use. Psychic derangements may appear when corticosteroids are used, ranging from euphoria, insomnia, mood swings, personality changes to severe depression.
Client Teaching & Education
Teach clients taking long-term prednisone therapy to never abruptly stop taking the medication and to report any adverse/side effects or new signs of infection.[18]
Glucocorticoid medication can cause immunosuppression, which makes it more difficult to detect signs of infection. Clients should seek advice from healthcare providers regarding vaccination administration while on glucocorticoids. Clients should report unusual swelling, weight gain, fatigue, bone pain, bruising, non-healing sores, visual and behavioral disturbances to the provider.
Use of glucocorticoid therapy may cause an increase in blood glucose levels. Clients should be advised to consume diets that are high in protein, calcium, and potassium.
Prednisone, Methylprednisolone, Hydrocortisone, and Fludrocortisone Medication Card
Now let’s take a closer look at the medication card comparing different formulations of corticosteroids.[19],[20],[21],[22], [23]
These example cards are intended to assist students to learn key points about each medication. Because information about medication is constantly changing, nurses should always consult evidence-based resources to review current recommendations before administering specific medication. Basic information related to each class of medication is outlined below.
Medication Card 9.4.1: Comparison of Prednisone, Methylprednisolone, Hydrocortisone, and Fludrocortisone (Corticosteriod Medications)
Therapeutic Effects
- Corticosteroids are used as replacement therapy in adrenal insufficiency, as well as for the management of various dermatologic, ophthalmologic, rheumatologic, pulmonary, hematologic, and gastrointestinal (GI) disorders. In respiratory conditions, systemic corticosteroids are used for the treatment of acute exacerbations of chronic obstructive pulmonary disease (COPD) and severe asthma.
- Mineralocorticoids are primarily involved in the regulation of electrolyte and water balance.
- Glucocorticoids are predominantly involved in carbohydrate, fat, and protein metabolism and also have anti-inflammatory, immunosuppressive, anti-proliferative, and vasoconstrictive effects.
| Class | Prototypes | Administration Considerations | Therapeutic Effects | Adverse/Side Effects |
|---|---|---|---|---|
| Glucocorticoid | Prednisone, Methylprednisolone |
|
Often used to reduce inflammation or for immunosuppression |
|
| Topical Glucocorticoid | Hydrocortisone cream |
|
Cream: topical relief of itching, redness, and swelling |
|
| Mineralocorticoids | Fludrocortisone |
|
Aldosterone replacement in Addison’s disease | Potential adverse effects from retention of sodium and water: hypertension, edema, cardiac enlargement, congestive heart failure, potassium loss, and hypokalemic alkalosis |
A client in a long-term care facility who has COPD receives prednisone 10 mg daily to help manage her respiratory status. Upon reviewing the client’s chart, the nurse notices that the client was diagnosed with osteoporosis in the past, but is not currently receiving medications indicated for osteoporosis. The nurse is concerned because the client requires assistance and is a fall risk so the nurse plans to call the provider.
- What cues in the client’s medical history cause the nurse to be concerned about the risk for a fracture?
- What medication(s) may be prescribed concurrently with prednisone to reduce the risk for a fracture?
- What other client teaching can the nurse provide to help reduce the client’s risk for a fracture?
- Bedside glucose testing with sliding scale insulin is ordered for this client, although she has no history of diabetes mellitus. What is the rationale for these orders?
- What cues would cause the nurse to contact the provider with the hypothesis that adrenal suppression is occurring?
Note: Answers to the activities can be found in the “Answer Key” sections at the end of the book.
Image Description
Figure 9.4a The Adrenal Gland and Associated Hormones
Illustration showing enlarged view of adrenal gland on top of the superior surface of kidney labelling the the cortex and medulla, and micrograph cross section of tissues:
| Tissue area | Hormones released | Examples |
|---|---|---|
| Connective tissue capsule | n/a | n/a |
| Zona glomerulosa (adrenal cortex) | Mineralocorticoids (regulate mineral balance) | Aldosterone |
| Zona fasciculata (adrenal cortex) | Glucocorticoids (regulate glucose metabolism) | Cortisol, Corticosterone, Cortisone |
| Zona reticularis (adrenal cortex) | Androgens (stimulate masculinization) | Dehydroepiandrosterone |
| Adrenal medulla | Stress hormones (stimulate sympathetic ANS) | Epinephrine Norepinephrine |
- "1818 The Adrenal Glands.jpg" by OpenStax is licensed under CC BY 4.0 Access for free at https://openstax.org/books/anatomy-and-physiology/pages/17-6-the-adrenal-glands ↵
- This work is a derivative of Anatomy and Physiology by OpenStax licensed under CC BY 4.0. Access for free at https://openstax.org/books/anatomy-and-physiology/pages/1-introduction ↵
- This work is a derivative of Daily Med by U.S. National Library of Medicine in the public domain. ↵
- Nieman, L., Biller, B., Findling, J., Murad, M., Newell-Price, J., Savage, M, & Tabarin, A. (2015, August 1). Treatment of Cushing’s Sydnrome: an endocrine clinical practice guideline. The Journal of Clinical Endocrinology & Metabolism, 100(8). pp. 2807-2831. https://academic.oup.com/jcem/article/100/8/2807/2836065 ↵
- Liu, D., Ahmet, A., Ward, L., et al (2013). A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy, Asthma & Clinical Immunology, 9, 30. https://aacijournal.biomedcentral.com/articles/10.1186/1710-1492-9-30 ↵
- Forciea, B. (2015, May 12). Anatomy and Physiology: Endocrine System: ACTH (Adrenocorticotropin Hormone) V2.0. [Video]. YouTube. All rights reserved. Video used with permission. https://youtu.be/4m7XflJzm2w. ↵
- Liu, D., Ahmet, A., Ward, L., Krishnamoorthy, P., Mandelcorn, E., Leigh, R., Brown, J., Cohen, A., & Kim, H. (2013, August 15). A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy, Asthma & Clinical Immunology, 9(30). https://doi.org/10.1186/1710-1492-9-30 ↵
- Liu, D., Ahmet, A., Ward, L., Krishnamoorthy, P., Mandelcorn, E., Leigh, R., Brown, J., Cohen, A., Kim, H. (2013, August 15). A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy, Asthma & Clinical Immunology, 9(30). https://doi.org/10.1186/1710-1492-9-30 ↵
- Liu, D., Ahmet, A., Ward, L., Krishnamoorthy, P., Mandelcorn, E., Leigh, R., Brown, J., Cohen, A., Kim, H. (2013, August 15). A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy, Asthma & Clinical Immunology, 9(30). https://doi.org/10.1186/1710-1492-9-30 ↵
- This work is a derivative of Daily Med by U.S. National Library of Medicine in the public domain. ↵
- Liu, D., Ahmet, A., Ward, L., Krishnamoorthy, P., Mandelcorn, E., Leigh, R., Brown, J., Cohen, A., & Kim, H. (2013, August 15). A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy, Asthma & Clinical Immunology, 9(30). https://doi.org/10.1186/1710-1492-9-30 ↵
- "Fluticasone.JPG" by James Heilman, MD is licensed under CC BY-SA 3.0 ↵
- "Methylprednisolone vial.jpg" by Intropin is licensed under CC BY 3.0 ↵
- "006035339lg Prednisone 20 MG Oral Tablet.jpg" by NLM is licensed under CC0 ↵
- This work is a derivative of Daily Med by U.S. National Library of Medicine in the public domain. ↵
- This work is a derivative of Daily Med by U.S. National Library of Medicine in the public domain. ↵
- Liu, D., Ahmet, A., Ward, L., Krishnamoorthy, P., Mandelcorn, E., Leigh, R., Brown, J., Cohen, A., & Kim, H. (2013, August 15). A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy, Asthma & Clinical Immunology, 9(30). https://doi.org/10.1186/1710-1492-9-30 ↵
- This work is a derivative of Daily Med by U.S. National Library of Medicine in the public domain. ↵
- This work is a derivative of Daily Med by U.S. National Library of Medicine in the public domain. ↵
- AHFS Patient Medication Information [Internet]. Bethesda (MD): American Society of Health-System Pharmacists, Inc.; c2019. Neomycin, Polymyxin, Bacitracin, and Hydrocortisone Topical; [reviewed 2018 Jun 15]. https://medlineplus.gov/druginfo/meds/a601061.html ↵
- Bornstein, S., Allolio, B., Arlt., W., Barthel., A., Don-Wauchope, A., Hammer, G., Husebye, E., Merke, D., Murad, M., Stratakis, C., & Tropy, D. (2016, February 1). Diagnosis and treatment of primary adrenal insufficiency: an endocrine society clinical practice guideline. The Journal of Clinical Endocrinology & Metabolism, 101(2). pp. 364-389. https://doi.org/10.1210/jc.2015-1710 ↵
- Nieman, L., Biller, B., Findling, J., Murad, M., Newell-Price, J., Savage, M, & Tabarin, A. (2015, August 1). Treatment of Cushing’s Sydnrome: an endocrine clinical practice guideline. The Journal of Clinical Endocrinology & Metabolism, 100(8). pp. 2807-2831. https://academic.oup.com/jcem/article/100/8/2807/2836065 ↵
- Liu, D., Ahmet, A., Ward, L., Krishnamoorthy, P., Mandelcorn, E., Leigh, R., Brown, J., Cohen, A., & Kim, H. (2013, August 15). A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy, Asthma & Clinical Immunology, 9(30). https://doi.org/10.1186/1710-1492-9-30 ↵
The hypothalamus stimulates the release of ACTH from the pituitary, which then stimulates the adrenal cortex to produce the hormone cortisol and steroid hormones important for the regulation of the stress response, blood pressure and blood volume, nutrient uptake and storage, fluid and electrolyte balance, and inflammation.
Neuroendocrine tissue composed of postganglionic sympathetic nervous system (SNS) neurons that are stimulated by the autonomic nervous system to secrete hormones epinephrine and norepinephrine.
The pattern in which the body responds in different ways to stress: The alarm reaction (otherwise known as the “fight or flight response,” the stage of resistance, and the stage of exhaustion).
Hormones released by the adrenal cortex that regulate body minerals, especially sodium and potassium, that are essential for fluid and electrolyte balance. Aldosterone is the major mineralocorticoid.
A mineralocorticoid, released by the adrenal cortex, that controls fluid and electrolyte balance through the regulation of sodium and potassium.

