3.4.2 – Grignard Reactions with Carbonyls
The Grignard reaction is an organic reaction used to form new carbon—carbon bonds by adding an alkyl or aryl group to an aldehyde or ketone carbon center. This results in an alcohol product.
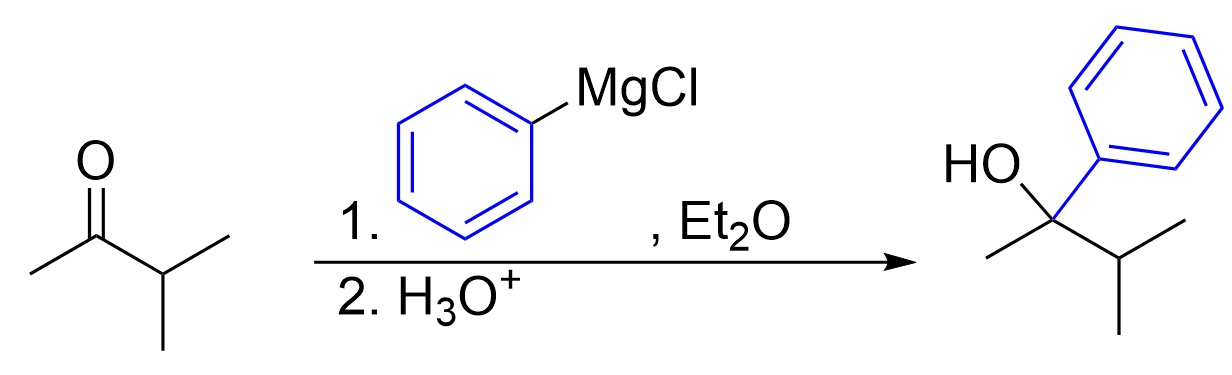
The Grignard Reagent
Before a Grignard reaction can be done, the Grignard reagent must first be synthesized. A Grignard reagent is made by treating an alkyl halide with elemental magnesium, Mg, using diethyl ether (Et2O) as a solvent. The halogen in the alkyl halide is often Cl or Br. You do not need to know the exact mechanism of how this reaction occurs. However, it is important to understand that the Mg inserts itself between the carbon and halide, while donating its 3s electrons to carbon, reducing it to a carbanion. This reagent is often written as R–MgX.
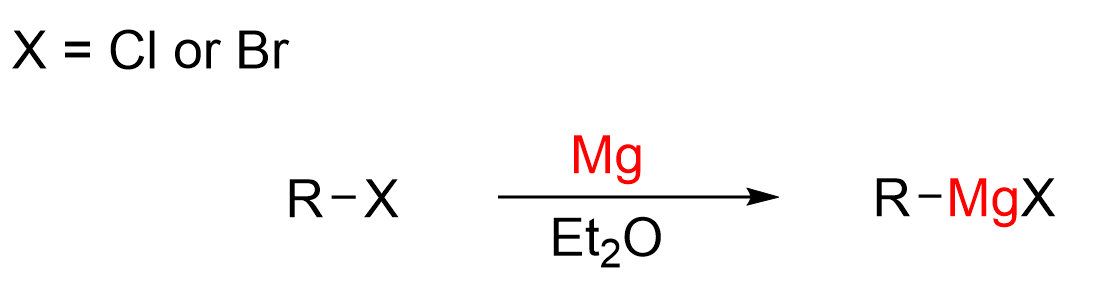
Magnesium is electropositive, with an electronegativity value of 1.31 on the Pauling scale. In comparison, carbon has an electronegativity of 2.02. As carbon is the more electronegative atom in the C–Mg bond, it exhibits a partial negative charge, whereas the Mg has a partial positive charge. Thus, this is one of the rare cases where the carbon acts as a nucleophile, rather than an electrophile. Another way to visualize this is with the carbon atom containing a lone pair of electrons and a formal negative charge, and write the Grignard reagent as an ionic salt, as shown below.

Reacting Carbonyls With a Grignard reagent
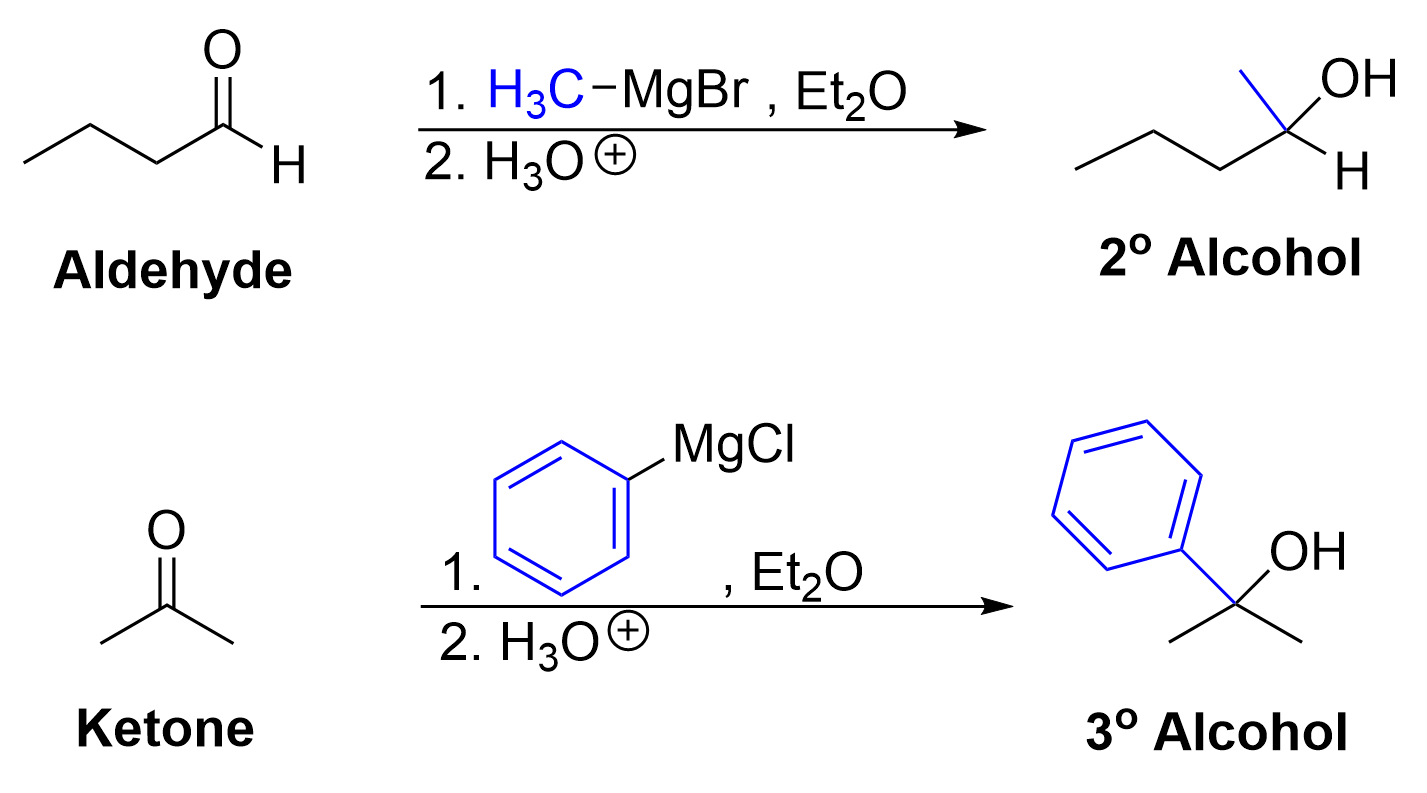
Grignard reagents react with aldehydes and ketones to form alcohols. The carbonyl group in the aldehyde or ketone contains an electrophilic carbon center due to the electronegative oxygen. Meanwhile, the Grignard reagent contains a nucleophilic carbon center. The nucleophilic Grignard reagent attacks the electrophilic carbonyl carbon atom, forming a new C–C bond while breaking a π bond to oxygen. Subsequent treatment with an acid delivers a proton (H+) to the oxygen, forming a new O–H bond, to produce an alcohol. The net result is breaking the C=O π bond, and forming two new σ bonds (C–C and O–H).
Reacting aldehydes and ketones with a Grignard reagent will form secondary and tertiary alcohols respectively.
(The full solution to this problem can be found in Chapter 5.2)
Mechanism of Grignard Reaction for Aldehydes and Ketones
The reaction consists of two individual mechanistic steps, which include the creation of the carbon—carbon σ bond and then the protonation of the alkoxide intermediate across the carbonyl π bond.
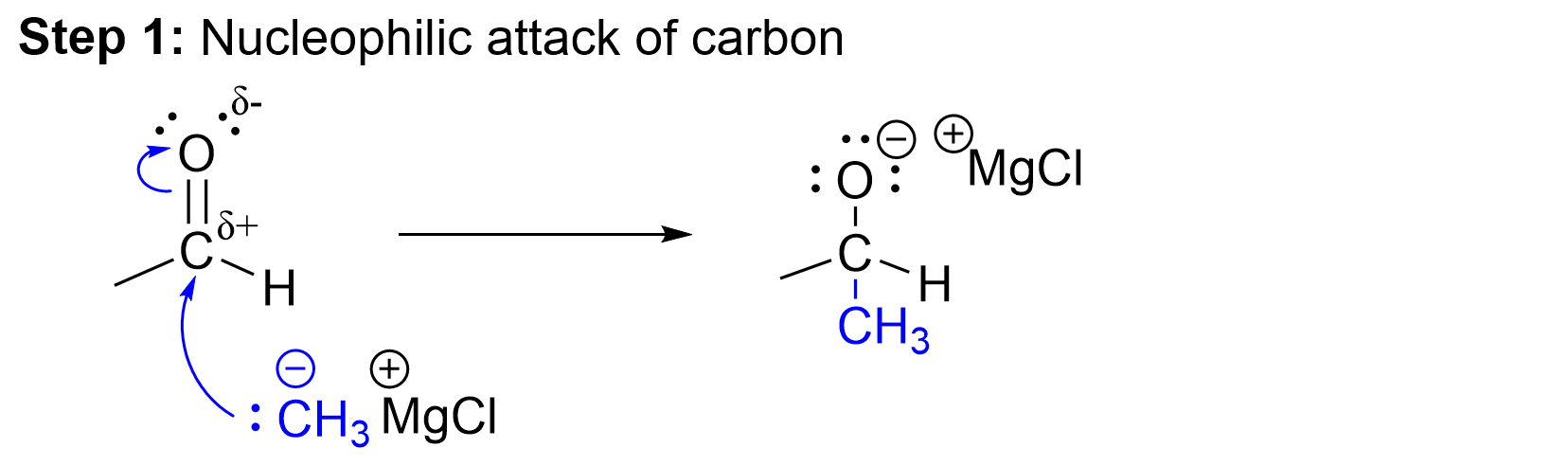
The first step of this reaction is the nucleophilic attack of the Grignard reagent at the electrophilic carbon centre of the carbonyl group. The lone pair of electrons on the Grignard reagent attack the carbon center of the aldehyde to form a new covalent bond. As a new bond is forming, another bond must break to ensure that the octet rule is not violated at carbon. The π bond preferentially breaks, with the electron pair from the π orbital of the C=O bond moving towards the electronegative oxygen. This step results in the formation of a new C–C bond and formation of an alkoxide intermediate with a formal negative charge at oxygen.
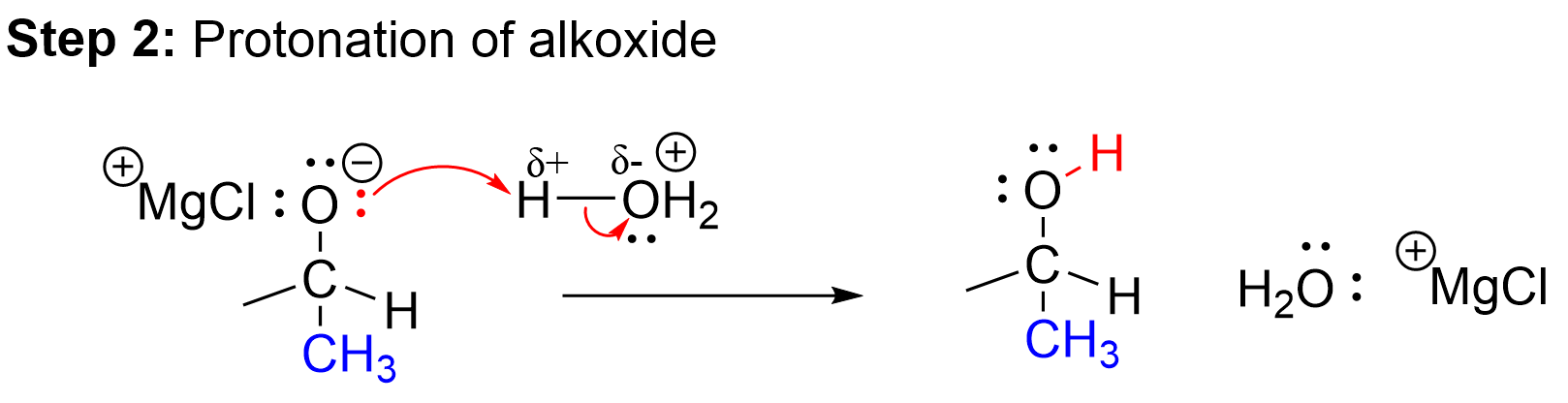
The second step of this reaction is the protonation of the negatively charged oxygen in the alkoxide intermediate. As the previous step ended with an anionic alkoxide as the intermediate, it must be protonated to stabilize the molecule and produce a neutral alcohol. This will occur by using acid, written as H3O+. The negatively charged oxygen uses a lone pair of electrons to abstract the proton on the acid. This is essentially an acid-base reaction between H3O+ (the acid) and the anionic alkoxide (the base). This second step results in our final product, a secondary alcohol.
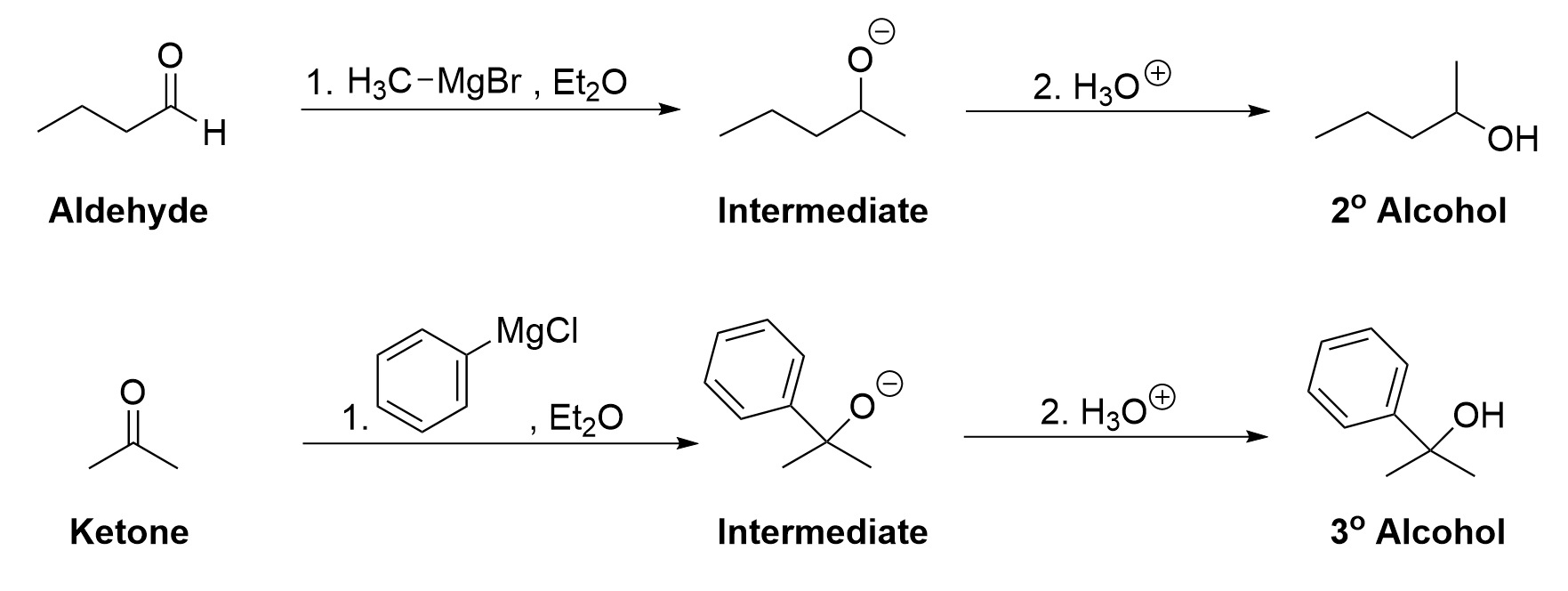
The addition of a Grignard reaction to an aldehyde produces a secondary alcohol. Aldehydes only contain one carbon attached to the central carbonyl carbon, but reaction with the carbon-containing Grignard reagent will increase the number of carbons attached to the central carbon to two.
The mechanism for Grignard reaction with ketones is functionally identical to that of aldehydes. It follows the same pattern of an initial nucleophilic attack from the Grignard reagent, followed by protonation of the anionic oxygen. Reacting a ketone with a Grignard reagent produces a tertiary alcohol. Ketones contain two carbons attached to the central carbonyl carbon, so reacting with the carbon-containing Grignard reagent will increase the number of carbons attached to the central carbon to three.
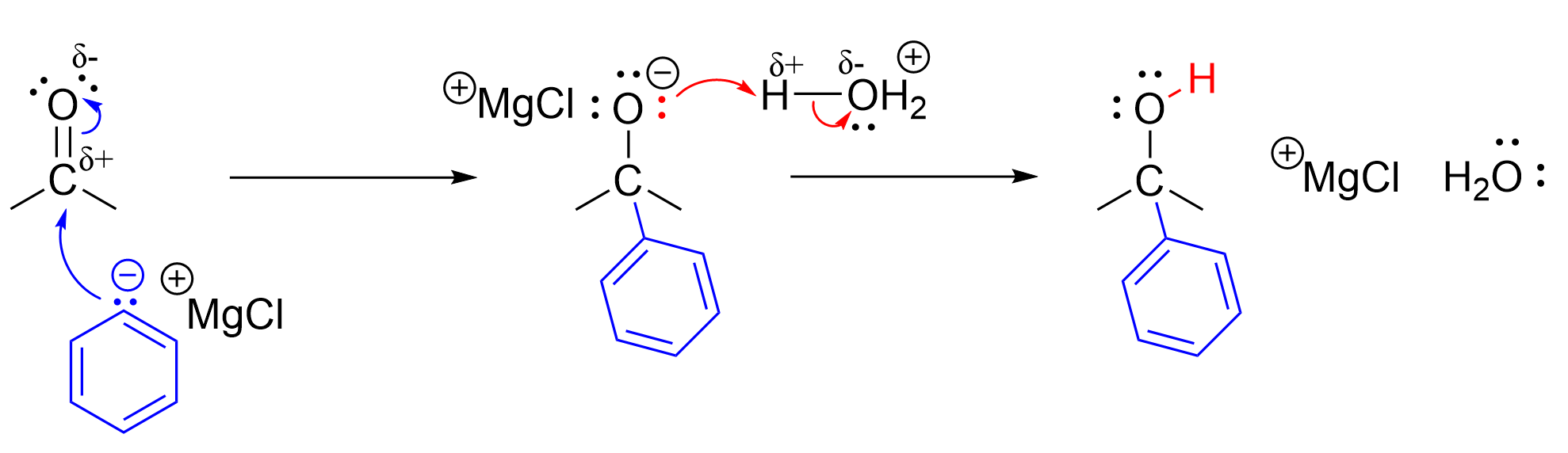
The two-step reaction can be summarized in the following manner, showing the intermediate as well as the starting reactant and product.
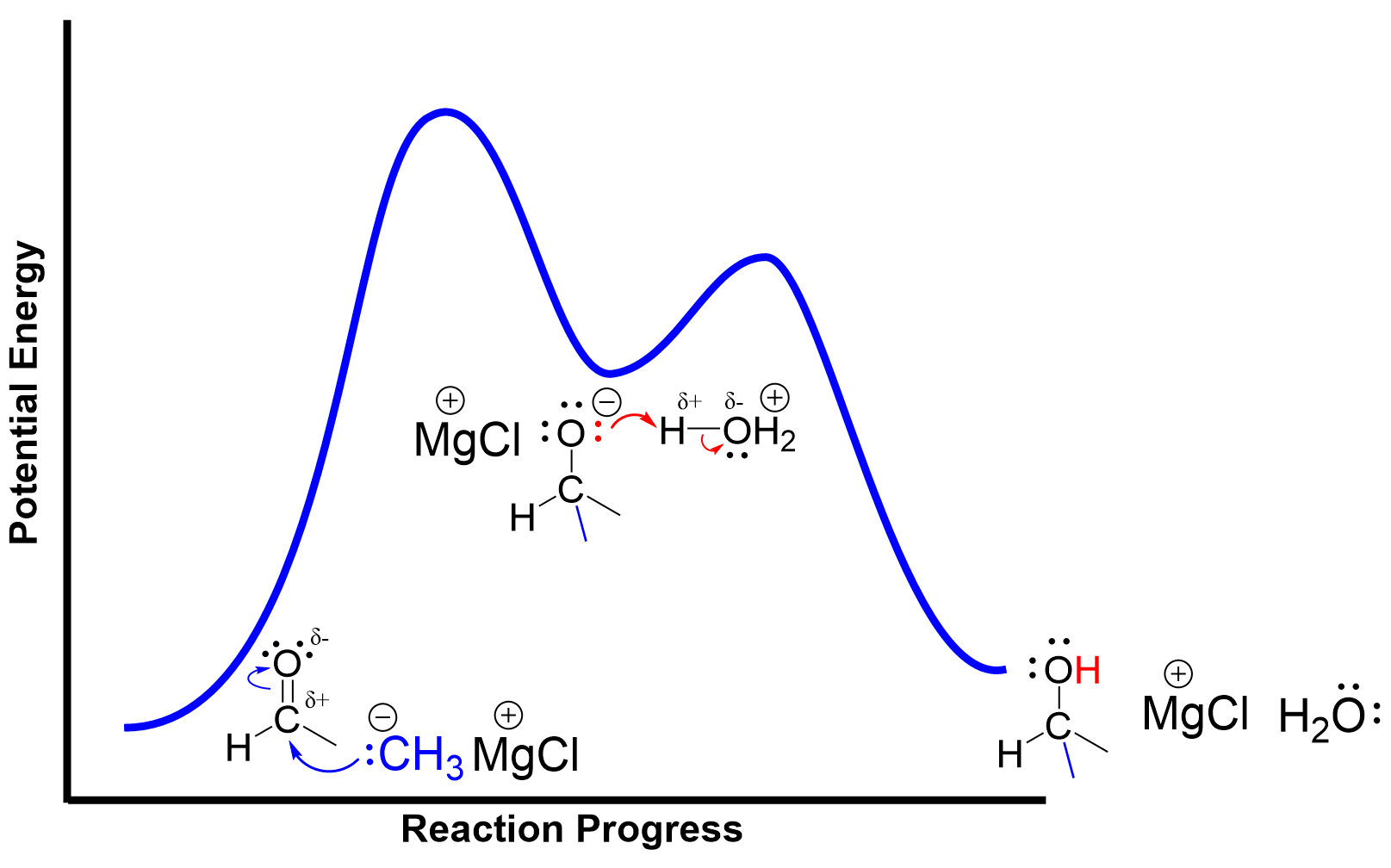
Energy Profile Diagram of Grignard Reactions
As this reaction follows a two-step process, we can draw an energy diagram to show the changes in energy and the intermediate formed in the reaction. The minimum of the energy profile represents the alkoxide intermediate that is synthesized in the first step after the addition of the C–C bond. The two maxima correspond to the two transition states where bond breaking and forming are occurring.
(The full solution to this problem can be found in Chapter 5.2)
Key Takeaways
- A Grignard reagent is a specific molecule which has a nucleophilic carbon. It has the general form R–MgX, where X is usually Br or Cl.
- Grignard reagents are formed when alkyl halides react with elemental magnesium in Et2O solvent.
- Grignard reagents can be used to form new carbon-carbon bonds when they are reacted with a carbonyl group containing reagent, as in an aldehyde or a ketone. This results in an alcohol product.
- There are two steps involved in the Grignard reaction with carbonyls:
-
- The nucleophilic Grignard carbon attacks the electrophilic carbon centre of the carbonyl group, breaking a π bond to oxygen in the process. This results in an alkoxide intermediate.
- The alkoxide intermediate is protonated in the presence of an acid to produce an alcohol.
- The degree of the alcohol product relies on how many carbons the carbonyl group was bound to initially.
Any feedback or comments on this chapter? You may either email chemoer@mcmaster.ca, access this MS Form, or provide a comment in the feedback box below.

