5.3 – Solutions for Chapter 4 – Chemical Biology
Chapter 4.3 – Aromaticity
1. Indicate the FALSE statement concerning aromatic compounds:
a) 1,3,5-Triacetylbenzene contains 12 π-electrons but is nevertheless aromatic.
b) The Hückel rule predicts that planar cyclic systems having (4n + 2) conjugated π ‑electrons are aromatic
c) Aromatic systems are planar in order to allow maximum overlap between adjacent p-orbitals.
d) π -bonds in aromatic systems are more reactive towards bromine than those in linear alkenes.
The correct answer is Option D. We will go through each statement one by one to determine how we reach this conclusion.
Option A is a true statement. The first thing you should do is draw out the compound. This compound contains a benzene ring with acetyl groups on positions 1, 3, and 5.
There are 3 rules to satisfy for a compound to be aromatic: cyclic, hybridization and Huckel’s rule.
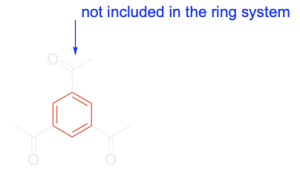
1. This compound is cyclic because benzene is cyclic, so we will only focus on the benzene portion. Note that the acetyl groups are not included in the ring system, so they will not be considered for the other 2 criteria.
2. This compound does contain sp2 –hybridization for all atoms that are in the ring system (shown in the blue highlight).
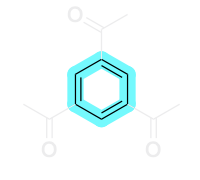
3. This compound does satisfy Huckel’s rule. For the atoms that are in the ring system, there are 6 π-electrons that satisfies the criteria for aromaticity as it is a number that can be generated from (4n+2).
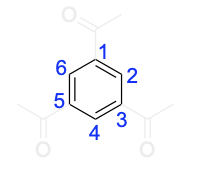
Therefore, 1,3,5–triacetylbenzene is aromatic.
Option B is a true statement. Huckel’s rule is one of the criteria used to evaluate the aromaticity of a compound. It states that if a planar and cyclic compound contains a number of electrons that satisfies the (4n+2) rule (which contains electron numbers in the order 2, 6, 10, 14, 18, 22, etc.,) it is considered aromatic.
Option C is a true statement. Aromatic systems must be planar. This is because the p–orbitals must be able to overlap to allow for delocalization of the electrons.
Option D is an FALSE statement, making Option D the correct answer. π-bonds in aromatic systems are not more reactive towards bromine than those in linear alkenes. This is because the π-bonds in aromatic systems are significantly more stable than in linear alkenes, so it is less reactive and will not undergo addition, halogenation or oxidation and reduction reactions like alkenes.
2. Which compound below is not aromatic? (You may assume that a compound is planar if it meets the other criteria for aromaticity.)
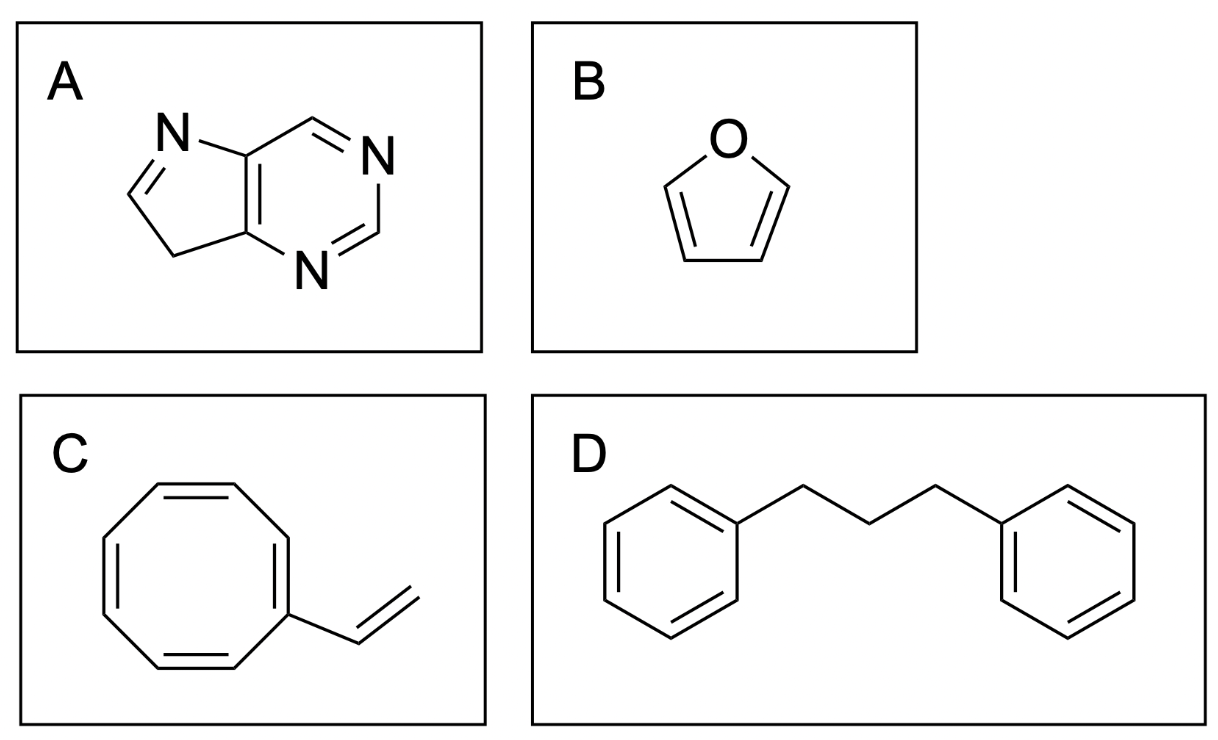
The correct answer is Option C, the third compound.
There are 3 rules to satisfy for a compound to be aromatic: cyclic, hybridization and Huckel’s rule.
All of the compounds contain cyclic portions (shown in red), while the portions that do not contribute to the ring system are highlighted in grey. Note that in the first molecule, there are 2 cyclic portions. The left-most portion is not included in the ring system because one of the atoms is sp3–hybridized, so it would not allow for the planar overlap of p–orbitals, and is not aromatic.
All atoms in the ring system in the 4 compounds can also be sp2 or sp–hybridized.
The last criteria to check for is Huckel’s rule.
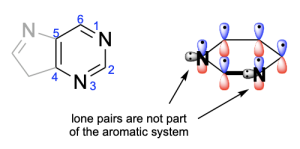
For the first compound, there are six π-electrons. This is a number that will satisfy Huckel’s (4n+2) rule. Note the none of the nitrogen atoms contribute a lone pair to the ring system since the p–orbital is already contributing 1 electron to the aromatic system. Therefore, this compound is aromatic.
In the second compound, there are also six π-electrons. This is a number that will satisfy Huckel’s (4n+2) rule. In this example, oxygen does contribute its lone pair to the π-system since there are no adjacent p–orbitals that can contribute 1 electron. Therefore, this compound is aromatic.
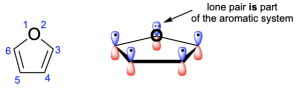
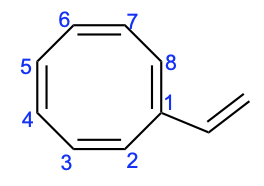
In the third compound, there are eight π–electrons. This is a number that will not satisfy Huckel’s (4n+2) rule, as there are 4n (8) electrons. Note that there is another double bond in this compound, but because it is not part of the cyclic ring system, it cannot contribute its electrons. Therefore, this compound is not aromatic.
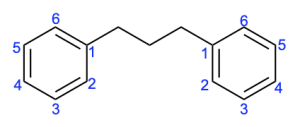
In the fourth compound, there are 2 cyclic systems, each with six π-electrons. This is a number that will satisfy Huckel’s (4n+2) rule. Note that you do not add up the number of electrons in each aromatic system to get 12 π-electrons, as this would suggest the compound is not aromatic. Since they are separate cyclic systems, you need to ensure that each ring meets this criteria, rather than looking at the sum. Therefore, this compound is aromatic.
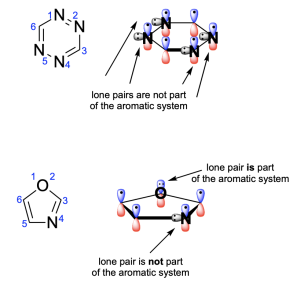
3. Which of the following statements about tetrazine and oxazole is FALSE?
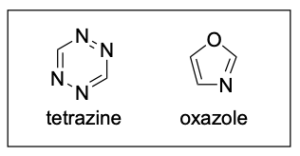
A. Both molecules are aromatic.
B. The oxygen atom of oxazole is sp3 hybridized.
C. Every nitrogen atom contributes 1 electron to the π-system.
D. Each molecule contains 6 delocalized electrons.
Option A is a true statement, making this an incorrect answer. To satisfy aromaticity, there are 3 criteria that must be met: cyclic, hybridization and Huckel’s rule. Both compounds are cyclic and contain atoms that are all sp2-hybridized. Additionally, the compounds satisfy Huckel’s rule and have six π-electrons. Note that none of the nitrogen atoms contribute their lone pairs to the π–system (as the p–orbitals contribute 1 electron), but the oxygen atom in oxazole does contribute its lone pair to the aromatic system. They are therefore both aromatic.
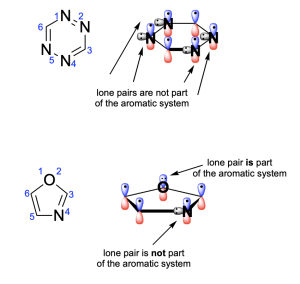
Option B is a false statement, making Option B the correct answer. The oxygen atom in oxazole is not sp3 hybridized. This is because the oxygen atom donates one of its lone pairs to the aromatic π–system. The oxygen atom is therefore involved in 2 bonds (both are to carbon atoms), and it only has one lone pair (since the other one is involved in the aromatic π–system). This creates an sp2 hybridized atom with trigonal planar geometry.
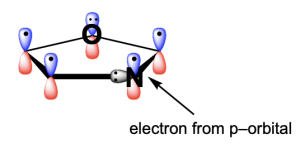
Option C is a true statement, making this an incorrect answer. As shown in Option A, nitrogen’s lone pair is not involved in the aromatic system and is instead perpendicular to the delocalized electrons in the p–orbitals. But, an electron from nitrogen’s p–orbital will be contributed to the aromatic system.
Option D is a true statement, making this an incorrect answer. As shown in Option A, the compounds have six π-electrons. Note that none of the nitrogen atoms contribute their lone pairs to the π–system (as the p–orbitals contribute 1 electron), but the oxygen atom in oxazole does contribute its lone pair to the aromatic system.