5.2 – Solutions for Chapter 3 – Reactivity
Chapter 3.6 – Synthesis
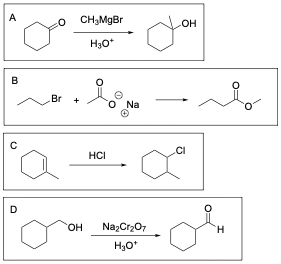
Option A is correct. When reacting a carbonyl–containing compound with a Grignard reagent in the presence of a proton source (such as hydronium), the carbon chain is elongated and the carbonyl is reduced to an alcohol.
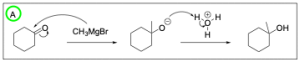
Option B is incorrect. When reacting an alkyl halide with a carboxylate, this is an SN2 reaction where the negatively charged oxygen will attack the electrophilic alkyl halide, kicking out the halide and creating an ester. While the product is an ester, it is not the correct product. The carbonyl portion of the carboxylate does not change, so there should be no chain elongation from that side. This is because the negatively charged oxygen is the nucleophile and will attack the electrophilic alkyl halide to grow the chain/undergo a reaction.
Option C is incorrect. This is a hydrohalogenation reaction where an alkene will attack an electrophilic hydrogen halide to form a new C–X bond. With unsymmetric alkenes, there are 2 products: the Markovnikov and Anti–Markovnikov product. The Markovnikov product is also known as the major product since it has a more favourable/stable carbocation intermediate. This product is the Anti–Markovnikov product, and while it is possible, it is not likely.

Option D is incorrect. This is an oxidation reaction involving a primary alcohol. Primary alcohols can be oxidized to aldehydes or carboxylic acids depending on the oxidizing agent used. PCC/CH2Cl2 is poisoned, so it will oxidize primary alcohols to aldehydes, while stronger oxidizing agents like KMnO4 or NaCr2O7 will oxidize a primary alcohol to a carboxylic acid. This reaction scheme shows a primary alcohol being oxidized to an aldehyde with a strong oxidizing agent which will not occur. Instead, a carboxylic acid would be produced. A poisoned oxidizing agent would be needed to obtain an aldehyde.

2. What is the product of the following reaction scheme?
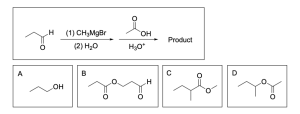
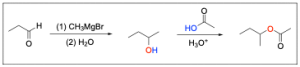
The correct answer is Option D. This multi–step synthesis question involves an aldehyde undergoing a Grignard reaction, and that product undergoing esterification. Whenever you see a synthesis question, it is best to draw out the mechanism to identify the product.
When the aldehyde undergoes a Grignard reaction, an alcohol with an elongated carbon chain will form.
The next step is acid–catalyzed esterification, where the alcohol and carboxylic acid join to form an ester under acidic conditions. The oxygen atom from the alcohol is the oxygen atom present in the ester (shown in red), while the other atoms form water as a by–product (shown in blue).
A breakdown of the products and intermediates is shown below.
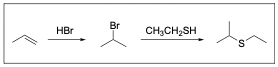
3. Thiols (R–SH) can act as a nucleophile. What is the expected final product (compound II) in this reaction?
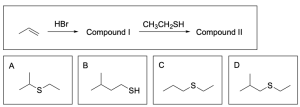
The correct answer is Option A. This multi–step synthesis question involves an alkene undergoing hydrohalogenation to yield a hydrogen halide (Compound I), while Compound I is then reacted with a thiol to yield a sulfide/thioether (Compound II). Whenever you see a synthesis question, it is best to draw out the mechanism to identify the product.
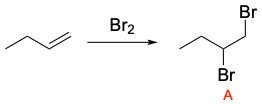
As previously stated, the first step is hydrohalogenation of the alkene to yield a hydrogen halide as shown below. Note that regioselectivity is a factor, and the Markovnikov product will be the major product (Compound I).
 The next step is reacting the alkyl halide with a thiol. Note that thiols behave very similar to alcohols due to similarities in properties between oxygen and sulfur. Therefore, the thiol will act nucleophilic in an SN2–like mechanism and will attack the electrophilic hydrogen halide to form a new C–S bond. Note that because the thiol used is neutral, an extra deprotonation step is required.
The next step is reacting the alkyl halide with a thiol. Note that thiols behave very similar to alcohols due to similarities in properties between oxygen and sulfur. Therefore, the thiol will act nucleophilic in an SN2–like mechanism and will attack the electrophilic hydrogen halide to form a new C–S bond. Note that because the thiol used is neutral, an extra deprotonation step is required.
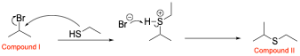
A breakdown of the products and intermediates is shown below.
4. What is product C, given the reaction sequence below?
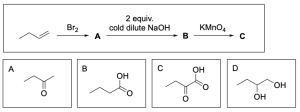
The correct answer is Option C. This multi–step synthesis question involves an alkene undergoing halogenation to yield a dibromoalkane (Compound A), while Compound A is then reacted with 2 equivalents of sodium hydroxide to yield a diol (Compound B). Compound B is then oxidized, which can yield a carboxylic acid or a ketone depending on if the alcohol is primary or secondary.
As previously stated, the first step is bromination of an alkene which will yield the dibromoalkane shown below.
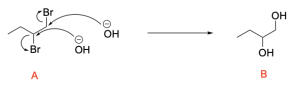
 The second step is to react Compound A with 2 equivalents of sodium hydroxide. This will react in an SN2 mechanism to form a diol.
The second step is to react Compound A with 2 equivalents of sodium hydroxide. This will react in an SN2 mechanism to form a diol.
The third step is to oxidize Compound B to form Compound C. Potassium permanganate is a strong oxidizing agent, so it will oxidize primary alcohols to carboxylic acids and secondary alcohols to ketones. The alcohol on the left is a secondary alcohol so it would be oxidized to a ketone. The alcohol on the right is a primary alcohol so it would be fully oxidized to a carboxylic acid.
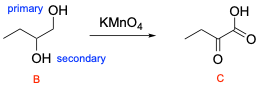
A breakdown of the products and intermediates is shown below.

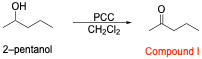
5. Reacting 2-pentanol with pyridinium chlorochromate (PCC) in dichloromethane generates compound I. Compound I is then reacted with ethylmagnesium bromide in diethyl ether, followed by aqueous acidic workup (H3O+) to generate compound II. What is the name of compound II?
A. ethyl 2-pentanoate
B. 3-methyl-3-hexanol
C. 3-heptanol
D. ethyl 2-pentyl ether
The correct answer to this question is Option D. The first thing you should do when you see multi-step synthesis questions without pictures is to draw out all of the information given to help you visualize the reagents. This is shown in the reaction scheme below.

The first step is oxidation of a secondary alcohol. The oxidizing agent used is poisoned, and the secondary alcohol will be oxidized to a ketone.
The second step is a Grignard reaction. The Grignard reagent will attack the ketone, which will grow the C–C skeleton and form a negatively charged oxygen. Hydronium is then introduced as a proton source to protonate the negatively charged oxygen and form an alcohol (Compound 2).
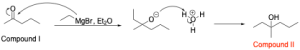
Using our knowledge of IUPAC nomenclature, we can name this compound. The highest priority functional group in this molecule is an alcohol at position 3, so the suffix will end in “–ol”. The longest chain present is 6 carbon atoms long, so the prefix will be “hex–”, yielding “3–hexanol”. Lastly, there is a methyl substituent at position 3, yielding the branch name “3–methyl”. Putting these 2 names together, the final name of Compound II is “3–methyl–3–hexanol”. Therefore, the correct answer is Option D.

6. Which reagents are needed to carry out the following synthesis?
A. (i) CH3MgBr/ether ; (ii) dilute HCl(aq)
B. (i) H2,Pt ; (ii) conc. H2SO4(aq)
C. (i) (1) NaBH4 (2) conc. H3O+(aq) ; (ii) conc. H2SO4(aq)
D. (i) (1) CH3MgBr/ether, (2) H3O+(aq) ; (ii) conc. H2SO4(aq)
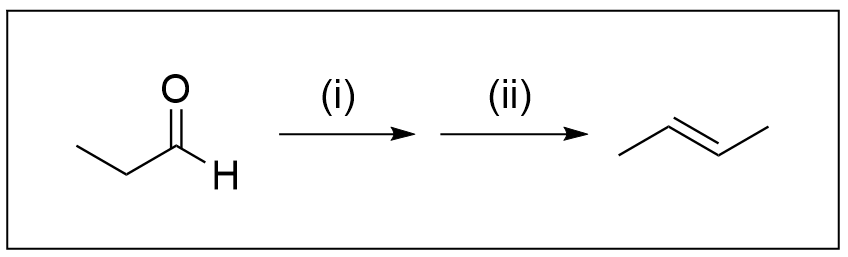
Option D is the correct answer. Our first step in a synthesis question, as always, is to count the carbons to see if a Grignard reaction occurred. It can be determined quickly that the starting molecule has 3 carbons, whereas the product has four. Thus, we know the Grignard reaction must have occurred in either step one or two. As Grignard reactions must occur with a carbonyl group, it must occur in step one. The reagents in a Grignard reaction are the Grignard reagent, which contains the added carbon(s) bonded to MgX, performed in ether. Since only one carbon has been added, the Grignard reagent must be CH3–MgX. Do not forget the second step of the Grignard reaction, which involves using H3O+ to protonate the alkoxide.
Using the process of elimination, only Option D remains as a possible answer to this question.
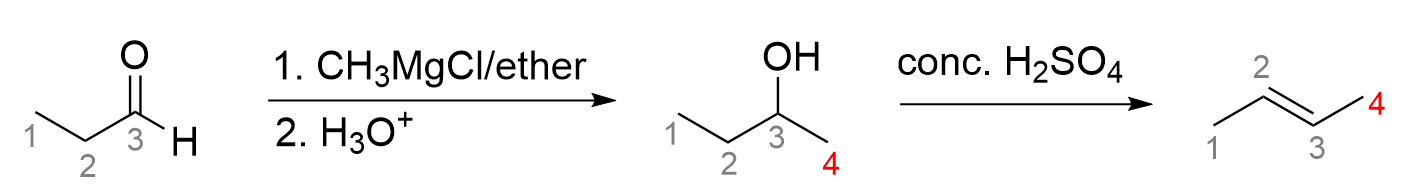
7. Which of the following statements about this 2-step reaction scheme is true?
A. The first step goes through an SN1 mechanism, and the second step requires PCC in CH2Cl2.
B. The first step goes through an SN2 mechanism, and the second step requires PCC in CH2Cl2.
C. The first step goes through an SN1 mechanism, and the second step requires KMnO4.
D. The first step goes through an SN2 mechanism, and the second step requires KMnO4.
The correct answer to this question is Option B.
The first part of each statement specifically speaks to the mechanism of nucleophilic substitution that occurs in the first step. The starting reagent is a primary alkyl halide, as the carbon bonded to the bromine only has one other bond to carbon. Because of this, this reaction occurs through an SN2 reaction mechanism. Recall that primary alkyl halides are more prone to undergoing through the SN2 pathway due to the steric effect. As there are no bulky alkyl groups blocking the electrophilic carbon, it is very easy for OH– to come around and attack it. This means options A and C are incorrect, as they state it reacts through an SN1 reaction, which tends to mainly happen with tertiary alkyl halide.
The second step involves the reagents in the reaction. Looking at the functional groups of the second and third molecules, it is clear an oxidation event has happened. The choices that we have are either KMnO4 or PCC in CH2Cl2. As the starting alcohol is a primary alcohol, the alcohol can either be oxidized to an aldehyde, which contains 2 bonds to oxygen, or a carboxylic acid, which contains 3 bonds to oxygen. As the final product is an aldehyde, this is not the most oxidized form, and thus, it calls for a more selective oxidizing agent. Recall that KMnO4 (as well as KCr2O7) always oxidizes to the most oxidized state (which would be either a carboxylic acid for primary alcohols or a ketone for secondary alcohols). On the contrary, PCC in CH2Cl2 is more selective and only oxidizes to an aldehyde or ketone. Thus, the correct option for this question is Option B.
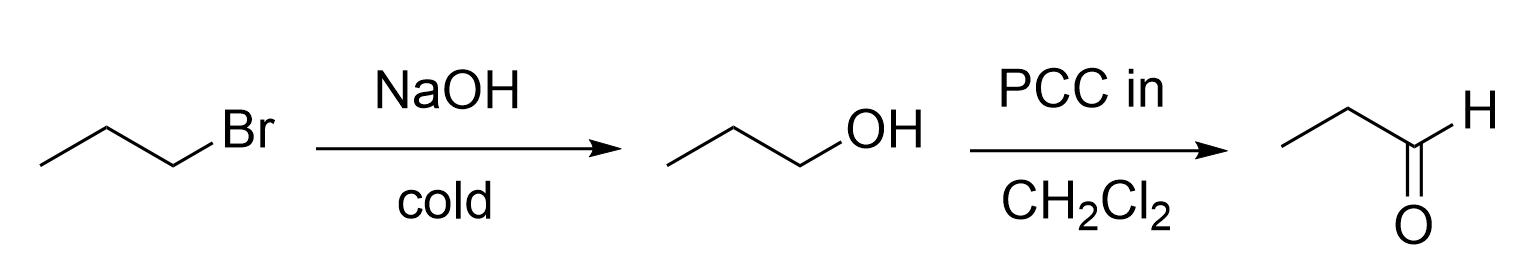
8. Which statement regarding the synthetic steps below is incorrect?
A. Step (iii) can be achieved by reaction with NaBH4 followed by addition of H3O+(aq).
B. Step (vi) can be achieved by reaction with H2O, and H3O+(aq)
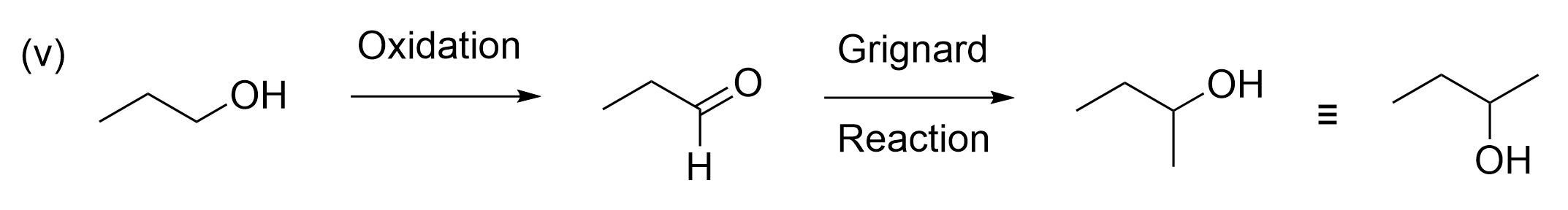
C. Step (v) can be achieved via an oxidation reaction, followed by a Grignard reaction and acid work-up.
D. Steps (ii) and (iv) can both be achieved by reaction with KMnO4(aq).
The correct answer to this question is Option B.
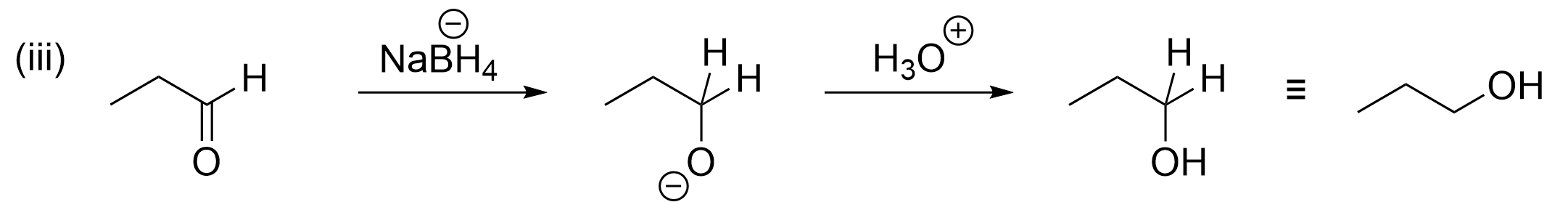
Option A is true, making this the incorrect answer. Step (iii) is a reduction reaction starting for an aldehyde and producing an alcohol. Reductions of carbonyl groups are performed using sodium borohydride (NaBH4), as discussed in Chapter 3.4.1, which donates a hydride to the electrophilic carbon. As this question is looking for the incorrect statement, this is an incorrect choice.
Option B is false, making it the correct answer to this question. Step (vi) is impossible to perform, as this involves the loss of one carbon. The starting reagent in this reaction contains four carbons, whereas the product contains three. We have not yet learned any reactions to lose carbon atoms, making this statement false.
Option 3 is also true, making it incorrect. As this is a multi-step reaction, we suggest that you write down the products at each step to see if it leads to the correct product. Following what the statement tells us, the starting alcohol can easily be oxidized to an aldehyde. As a carbonyl, it can then react with a Grignard reagent which adds another carbon onto the alkyl chain. This statement is true, as the final product in this stepwise reaction matches the product seen in the question. Thus, this is not the correct answer.
Option 4 is also true, making it an incorrect choice. Both starting products can be oxidized using KMnO4, which oxidizes to the most oxidized form. Thus, it is possible for an aldehyde to be oxidized to carboxylic acid, and a secondary alcohol to a ketone.
9. Which of the following statements about the reduction of an aldehyde by sodium borohydride is true?
A. The aldehyde acts as a nucleophile and the intermediate has a negative charge.
B. The aldehyde acts as an electrophile and the intermediate has a positive charge.
C. The aldehyde acts as an electrophile and the intermediate has a negative charge.
D. The aldehyde acts as a nucleophile and the intermediate has a positive charge.
The correct answer is Option D.
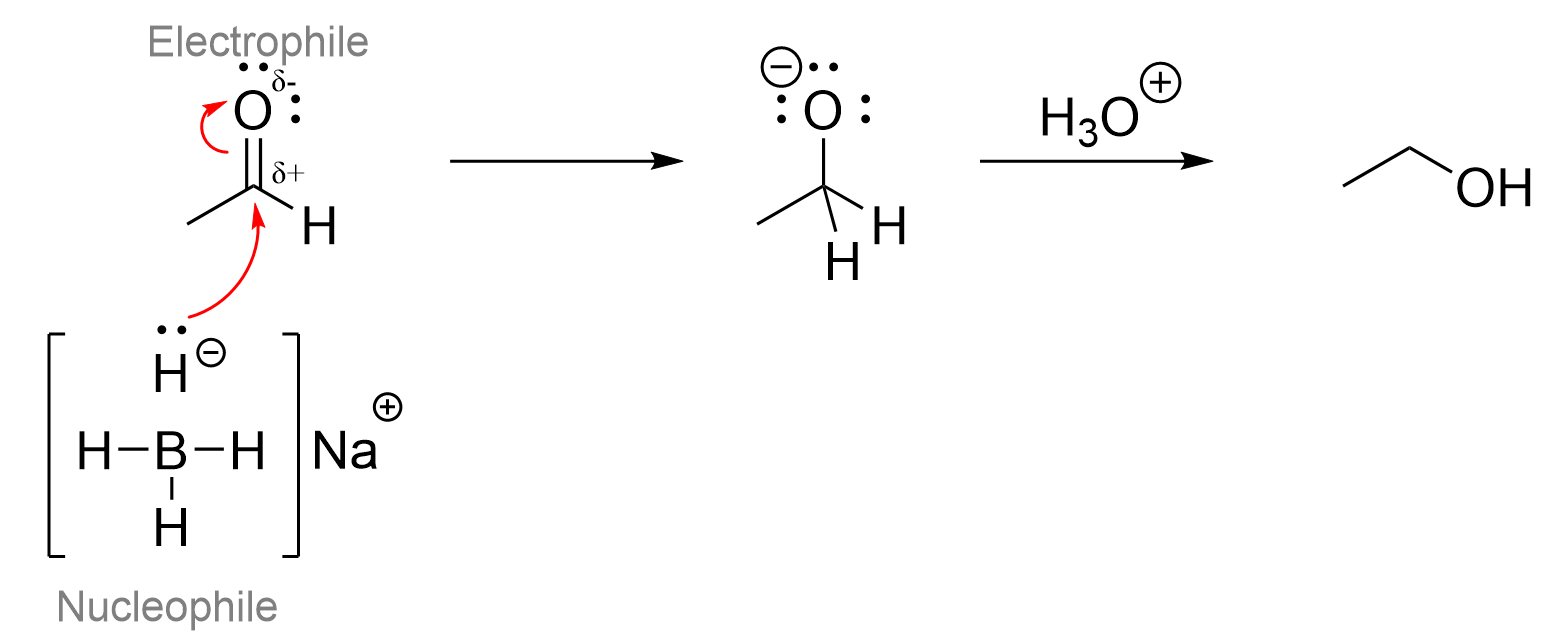
To visualize the process, we recommend drawing out the reduction mechanism of an aldehyde by sodium borohydride. Recall that carbonyls contain the polar bond between carbon and oxygen, giving carbon a partial positive charge due to the electronegativity of oxygen. As it contains a partial positive charge, the aldehyde acts as an electrophile.
As the hydride attacks the central carbon, it pushes the electrons from the double bond up towards the electronegative oxygen. This is to avoid the carbon exceeding 8 electrons in its valence shell. Thus, as the oxygen now contains an extra two electrons and loses one bond to carbon, it becomes negatively charge. This makes the correct answer to the question Option D.
For more information on sodium borohydride reductions, please consult Chapter 3.4.1,
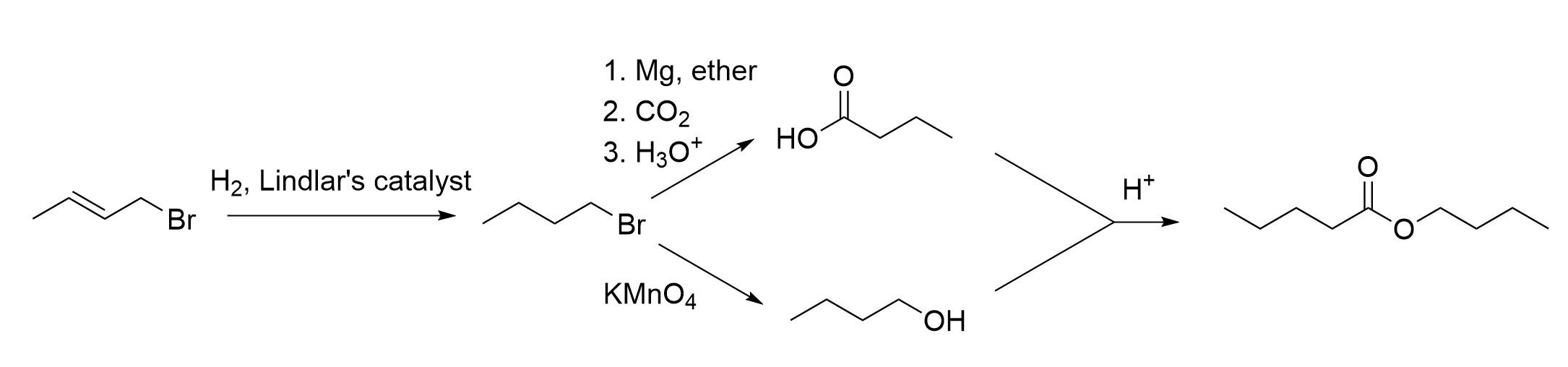
10. What reagents and conditions are needed for this synthetic scheme?
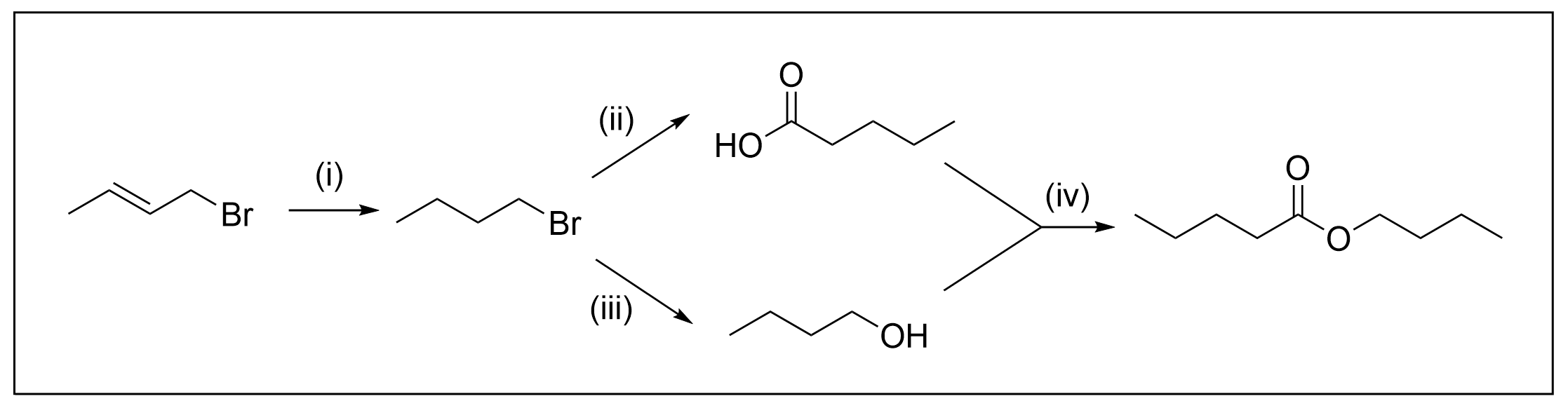
A. H2, Pd/C ; (ii) Mg, Et2O, CO2, H3O+ ; (iii) cold dilute NaOH ; (iv) H+
B. H2, Lindlar’s catalyst ; (ii) KMnO4 ; (iii) H2O ; (iv) H+
C. H2, Pt(s) ; (ii) KMnO4 ; (iii) H2O ; (iv) H3O+
D. H2, Pd/C ; (ii) PCC, CH2Cl2 ; (iii) H2O ; (iv) CH3OH, H+
The correct answer is Option A.
The first thing to do in questions with synthetic schemes is to identify the reactions that are occurring in each step. By knowing the reactions, it will make it much easier to determine what reagents are used at each step. You can do this by counting carbons and looking at the changes in functional groups. For example, step (i) shows an alkene becoming an alkane, which is achieved through hydrogenation. In step (ii), it can be seen that there is a fifth carbon added onto the alkyl chain, which means a Grignard reaction must have taken place to make a carboxylic acid. Step (iii) showcases a change from an alkyl halide to an alcohol, which we known is achieved through nucleophilic substitutions. Lastly, step (iv) shows a carboxylic acid and an alcohol reacting together to make an ester.
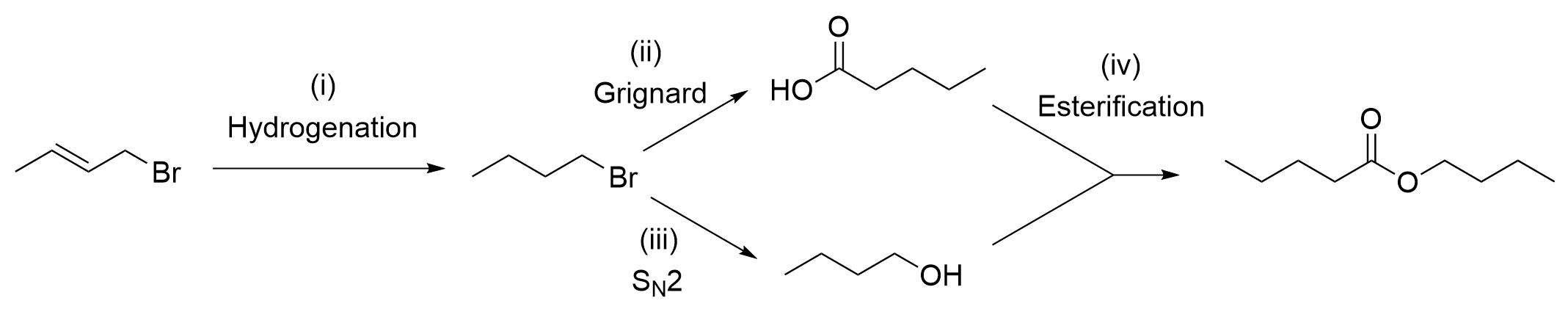
With this information, we can then determine the reagents used at each step. Hydrogenation always involves the uses of hydrogen gas (H2). Typically, a catalyst is used alongside H2, either a noble transition metal such as Pd or Lindlar’s catalyst. In this case, since we are going from an alkene to an alkane, it does not matter what type of catalyst used. This is only important if the reduction is from an alkyne to a cis alkene, in which Lindlar’s catalyst must be used. For more information, please refer to Chapter 3.2.3.
Step (ii), as we know, is Grignard reaction. This is because there are five carbons on the alkyl chain, whereas previously, there were only four. Although Grignard reactions require a carbonyl group (such as an aldehyde, ketone, or carbon dioxide CO2), recall the Grignard reagent also participates in the reaction. It is an alkyl chain containing the group –MgX, which gives the carbon its nucleophilic properties. Since this molecule is an alkyl halide, this can actually react with magnesium (Mg) to create a Grignard reagent (as seen in Chapter 3.4.2). To add a single carbon and make a carboxylic acid, this can then react with CO2 to make the desired product . Using the process of elimination, it is easy to determine the correct multiple choice answer from these two steps.
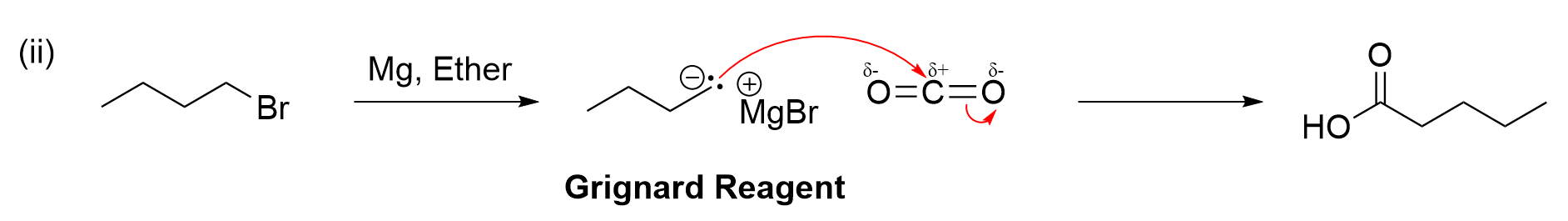
To fully understand the reaction schematic, the remaining steps will be explained. Step (iii) showcases a nucleophilic substitution reaction. As the bromine group is substituted with an alcohol group, this means OH– must be the nucleophile. Therefore, reagents that contain an OH group such as NaOH can react to form the desired product.
Step (iv) showcases an esterification reaction between a carboxylic acid and an alcohol. Recall that this is referred to as an acid-catalyzed esterification. This reaction requires the use of two catalysts: heat and concentrated acid, such as H2SO4. Thus, these are the two required reagents to form the final ester product. For more information, please refer to Chapter 3.5.
With each of the steps’ reagents, below shows the completed reaction schematic. This corresponds to Option A as the correct answer.