5.2 – Solutions for Chapter 3 – Reactivity
Chapter 3.5 – Reactions of Carboxylic Acids and Derivatives
1. Which of the following statements is false about the synthesis of carboxylic acid?
A. The carboxylic acid can be synthesized by either alcohol oxidation under K2Cr2O7 or reacting Grignard reagent with CO2.
B. When making carboxylic acid by reacting CO2 and Grignard reagent, the CO2 and H3O+ act as electrophiles.
C. When making carboxylic acid by reacting CO2 and Grignard reagent, the Grignard reagent and carboxylate anion act as nucleophiles.
D. When making carboxylic acid by reacting CO2 and Grignard reagent, the CO2 and carboxylate anion act as nucleophiles.
Option D is the correct answer to this question, as the statement is false. The easiest way to determine the validity of these statements is to draw out mechanism of carboxylic acid formation with CO2 and a Grignard reagent. In this reaction, the Grignard reagent acts as a nucleophile and attacks CO2, hence, this statement is false. Recall that the central carbon in CO2 is partially positive due to the electronegative oxygens. This makes it an electrophile in this step, as it is attacked by the nucleophilic Grignard reagent.
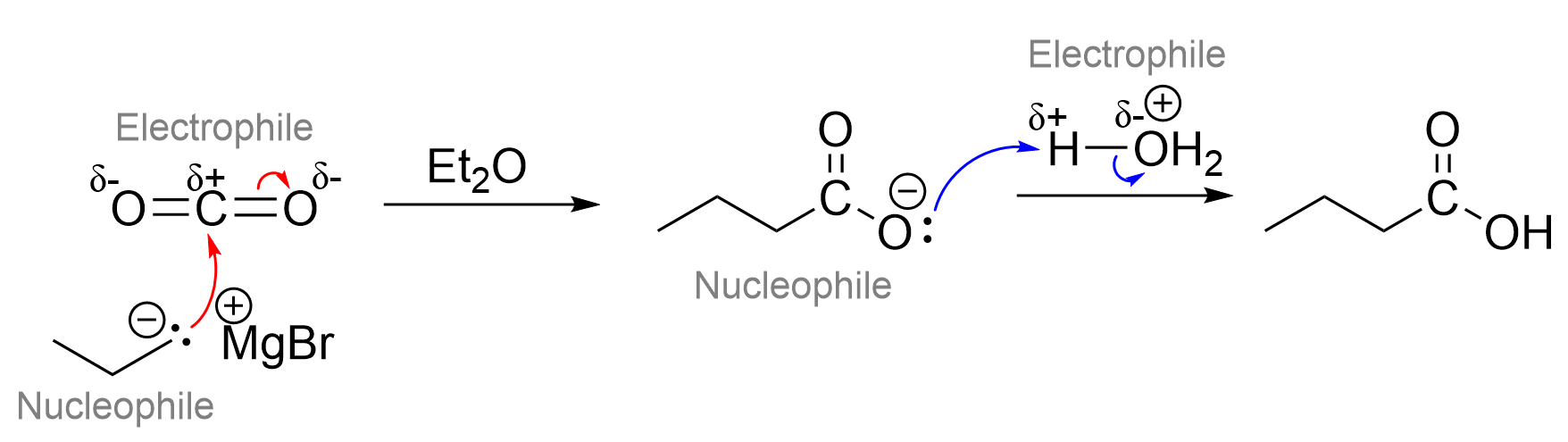
Answers A, B, and C are also true statements, meaning they are not the correct answer. CO2 can be oxidized by K2Cr2O7, a non-selective oxidizing agent, to a carboxylic acid. It can also react with a Grignard reagent (seen above) to form a carboxylic acid. In this reaction, the CO2 and H3O+ act as the electrophiles, as both have areas of electron-deficiency, as seen by the partial positive charges. On the contrary, the Grignard reagent and carboxylate anion act as nucleophiles due to the presence of lone pairs and negative charges.
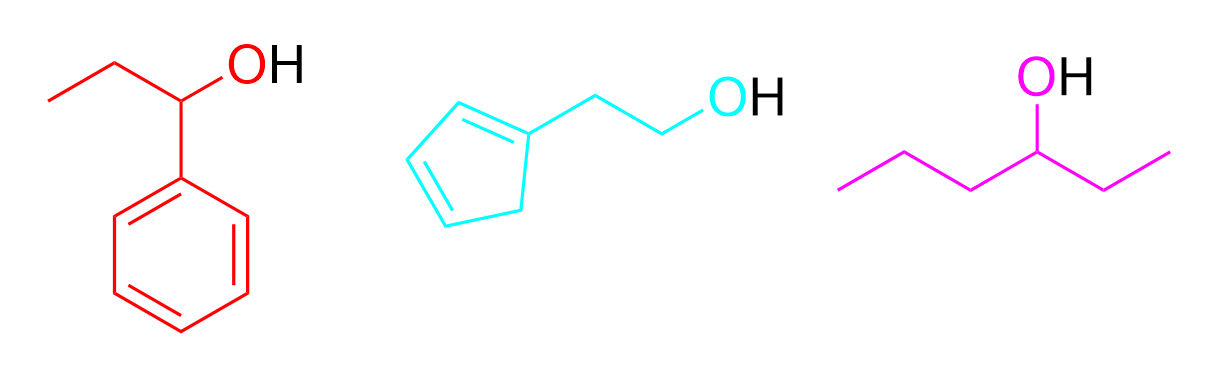
2. Given the following molecules in the system, which of following are possible products under heat and acidic conditions?
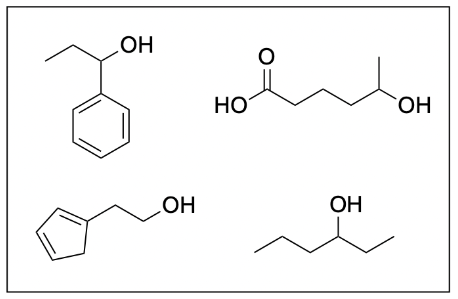
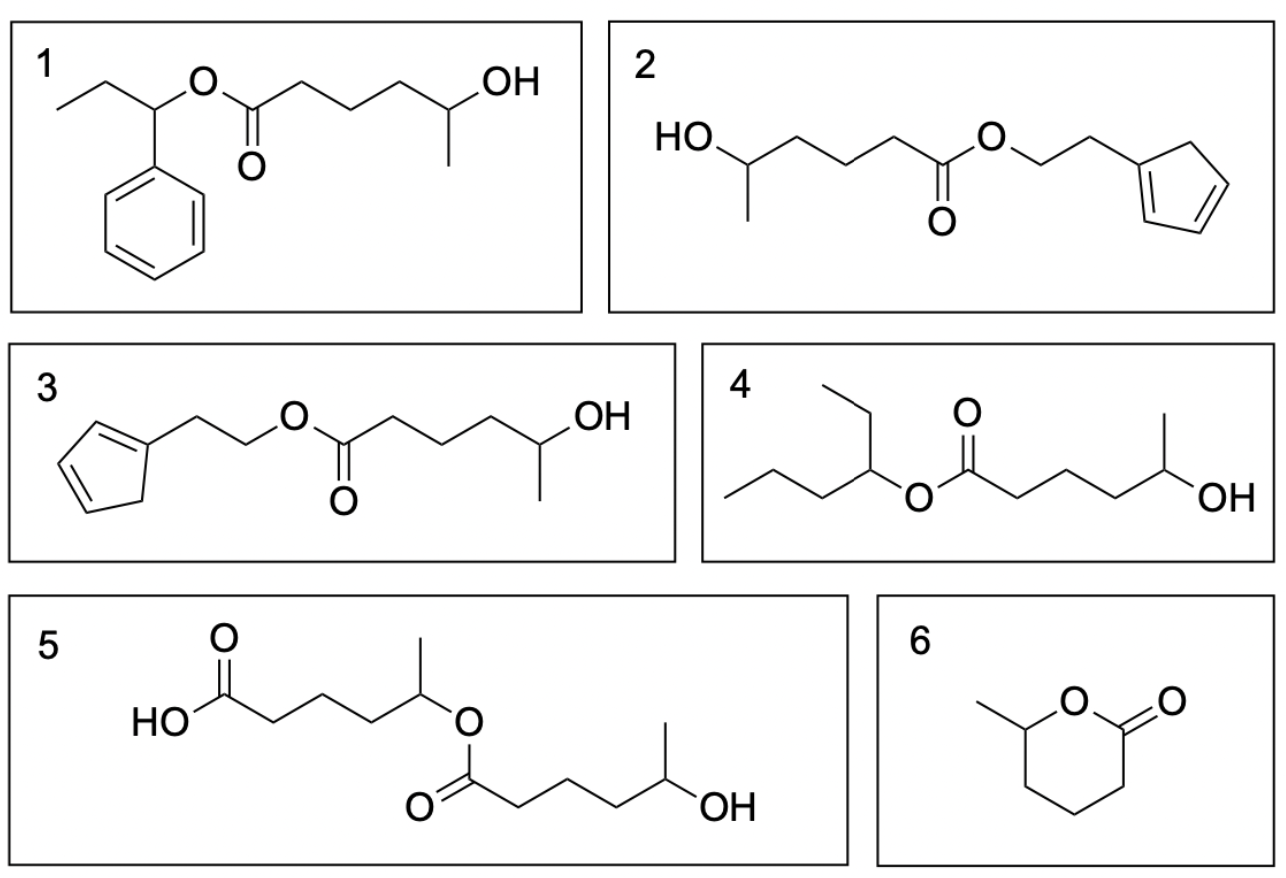
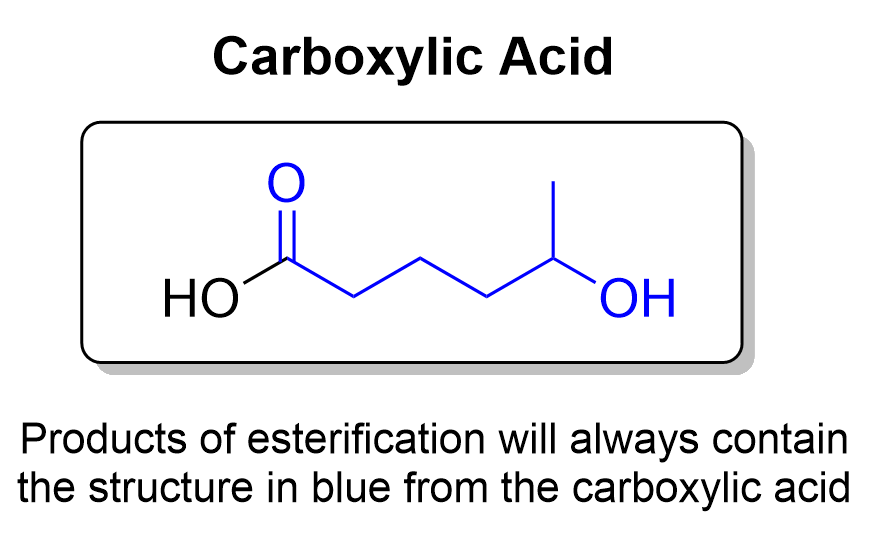
All six molecules are correct and can form under heat and acidic conditions. When determining the products of an acid-catalyzed esterification reaction, the first thing you should do is determine the structure of the carboxylic acid that will retain after esterification. This can allow you to quickly eliminate options that do not have the correct structure. As seen below, the structure in blue will be conserved in every ester product.
The other three molecules are alcohols, meaning they can react with the carboxylic acid to form an ester. Each compound can donate their alkyl chain to react and attach itself onto the end of the carboxylic acid. The coloured parts of the structure below indicate what will be added to the final product.
An easy way to determine whether the given options are real products is to cut through the COO (carboxylate) group and look at the carbon structure on each side. If one side matches with the carboxylic acid, and the other side matches with the carbon structure of the alcohol, then the product can be formed. For example, the first ester conserves its red carbon structure from the alcohol and the blue structure from the carboxylic acid. Thus, this is a possible product in this reaction. The same can be done for every other ester product, as shown below. Molecules 2 and 3 are the same molecule, except mirrored horizontally.
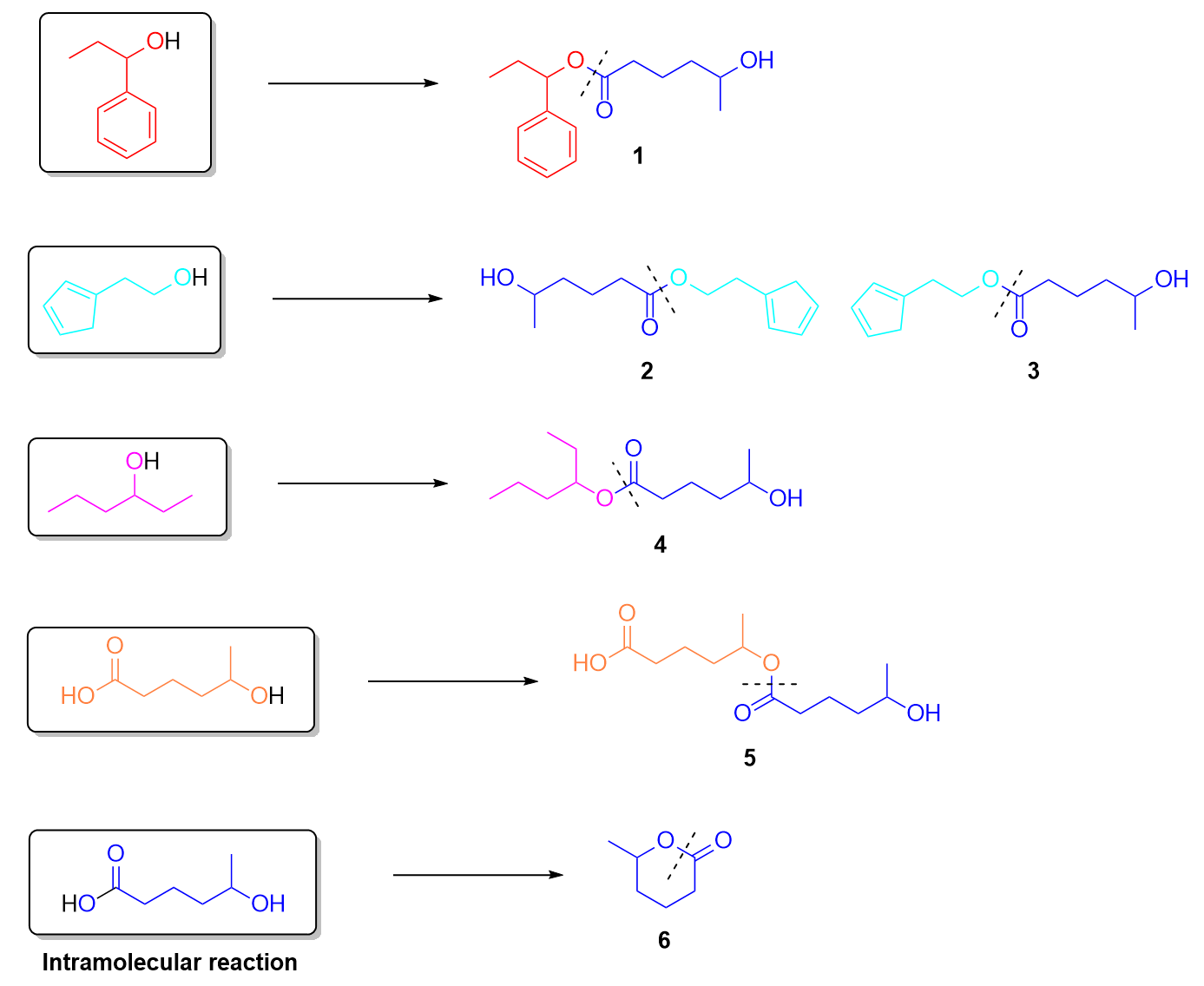
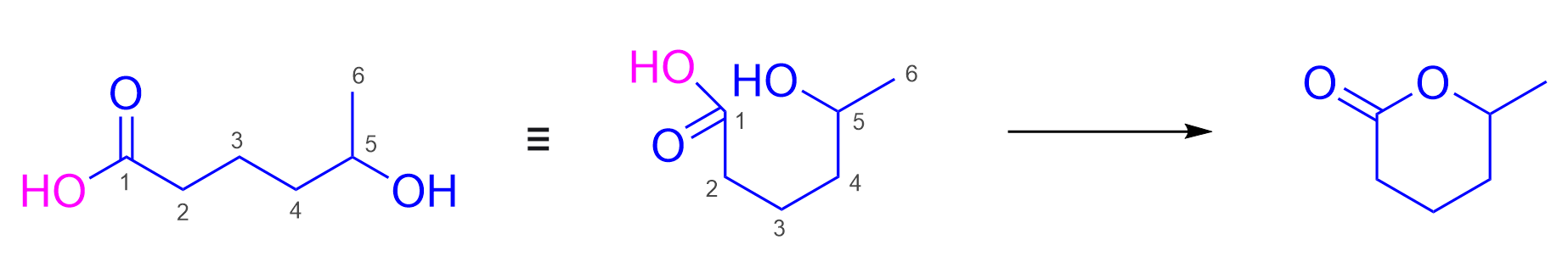
Molecules 5 and 6 may be difficult to identify as correct as these show the carboxylic acid reacting with itself. As the carboxylic acid contains an alcohol on the other side of the compound, it is possible for it to react with another molecule of itself to form Molecule 5. It can also react intramolecularly with itself to form a ring, as seen in Molecule 6. The ring may be difficult to visualize at first– one way to understand how it forms is to imagine the two -OH groups on each side of the carboxylic acid overlapping with each other to form the singular oxygen (found in between the two carbons). Refer to the image below for a visual explanation of the ring forming process.