5.2 – Solutions for Chapter 3 – Reactivity
Chapter 3.4.1 – Sodium Borohydride Reduction of Carbonyls
The correct answer to this question is Option C. Sodium borohydride is a reducing agent and will reduce a carbonyl to an alcohol by acting similar to the nucleophilic hydride ion. It will attack the electrophilic carbonyl carbon atom, which forms a C–H bond, while pushing electron density onto the oxygen, making it negatively charged. Adding hydronium provides a source of hydrogen atoms to protonate the negatively charged oxygen, yielding an alcohol.
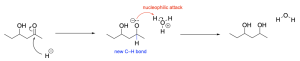
The intermediate in this reaction is present after sodium borohydride is introduced and before hydronium is introduced. These two steps are kept separate because the nucleophilic sodium borohydride would attack hydronium to protonate itself, leaving water as a by-product, and no reaction.
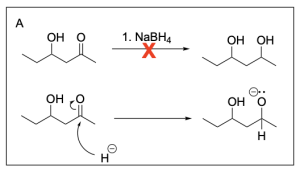
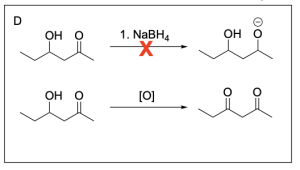
Option A is incorrect. This option illustrates the final product, not the intermediate product. The intermediate is an alkoxide, and sodium borohydride will not protonate the alkoxide. Instead, a proton source such as hydronium would need to be introduced after to obtain the final product.
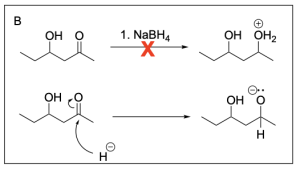
Option B is incorrect. Sodium borohydride is a reducing agent and will attack the electrophilic carbonyl carbon atom which pushes electron density onto the oxygen atom, generating a negatively charged alkoxide. There are no proton sources to protonate the oxygen atom, so the oxonium cation would not be generated, nor would it be an intermediate in this reaction.
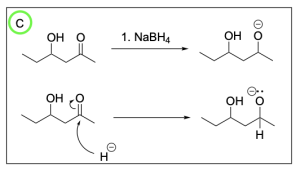
Option C is correct. Sodium borohydride is a reducing agent that will attack the electrophilic carbonyl carbon atom which pushes electron density onto the oxygen atom generating a negatively charged alkoxide as an intermediate.
Option D is incorrect. Sodium borohydride is a reducing agent, not an oxidizing agent. In addition, a ketone cannot be further oxidized to a carboxylic acid, as it would require the unfavourable breakage of a C–C bond. The secondary alcohol could be oxidized to a ketone, but the ketone could not be oxidized to a carboxylic acid.
2. Which of following statements about the mechanism is true?
A. Both (i) and (ii) are drawn correctly.
B. The step (ii) is drawn incorrectly.
C. The step (i) is drawn incorrectly.
D. Both (i) and (ii) are drawn incorrectly.
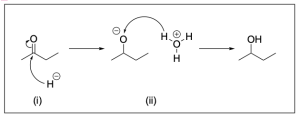
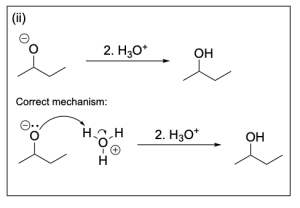
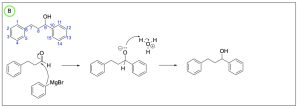
The correct answer to this question is Option B. This mechanism shows sodium borohydride reduction of carbonyls. Sodium borohydride is a reducing agent that will reduce a carbonyl group to an alcohol by acting similar to the nucleophilic hydride ion. It will attack the electrophilic carbonyl carbon atom, which forms a C–H bond while pushing electron density to the oxygen making it negatively charged. Adding hydronium provides a source of hydrogen atoms to protonate the negatively charged oxygen, yielding an alcohol.
Step (i) is drawn correctly. The nucleophilic hydride will attack the electrophilic carbonyl, pushing electron density onto the oxygen forming an alkoxide and a new C–H bond.
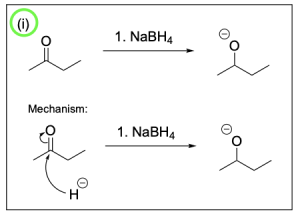
Step (ii) is drawn incorrectly. Hydronium is not nucleophilic and will not attack the alkoxide. Instead, the nucleophilic alkoxide will attack hydronium, which will protonate the alkoxide and form an alcohol and water. Chapter 3.1.1. discusses the direction of the arrows in organic reactions. The tail of the arrow should point to the electrophile while the head of the arrow should originate from the nucleophile. Therefore, Option B is the correct answer.
Chapter 3.4.2 – Grignard Reactions with Carbonyls
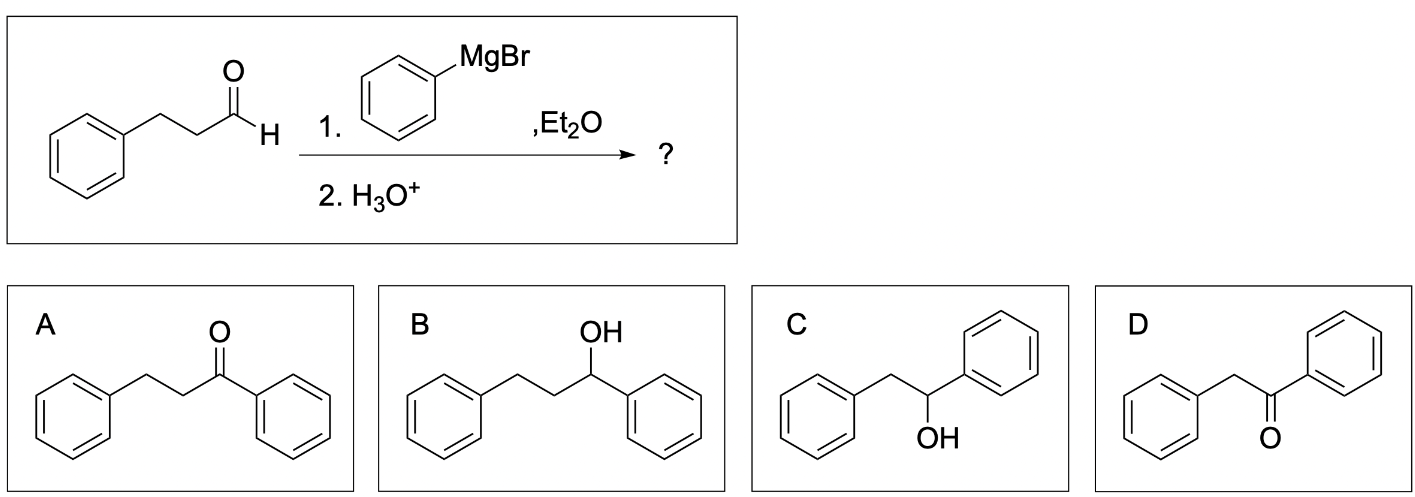
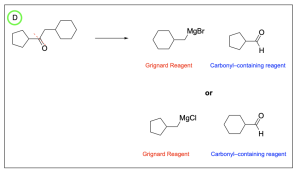
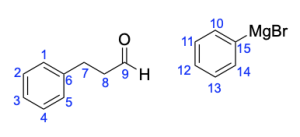
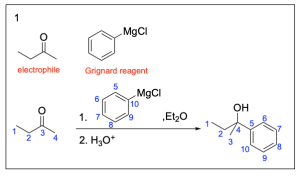
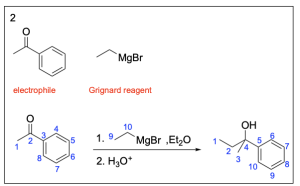
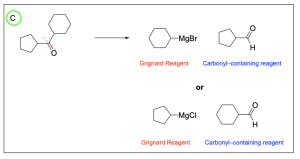
The correct answer to this question is Option B. This is a Grignard reaction, where the Grignard reagent (containing either magnesium chloride or magnesium bromide) is nucleophilic and attacks the electrophilic carbonyl forming new C–C bonds. The carbon atom adjacent to the magnesium bromide is nucleophilic and will perform the nucleophilic attack. Since Grignard reactions form new C–C bonds, the first thing you should do is count the number of carbon atoms in the carbonyl–containing compound and in the Grignard reagent, as this amount must add up to the number of carbon atoms in the product. There are 15 carbons in total from the reagents, so the product must contain 15 carbon atoms.
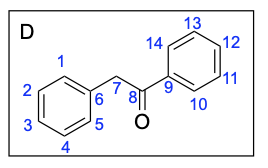
Option A is incorrect. While this compound contains the correct number of carbon atoms, a Grignard reaction reduces a carbonyl to an alcohol by forming a new C–C bond. This compound is fully oxidized and is not reduced. To obtain this compound after a Grignard reaction, you would need to add an oxidizing agent (such as PCC/CH2Cl2).
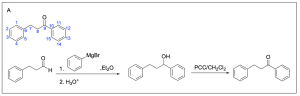
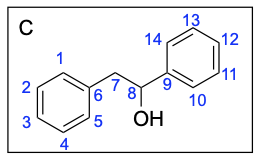
Option B is correct. A Grignard reaction reduces a carbonyl to an alcohol by forming a new C–C bond. This compound also contains the correct number of carbon atoms.
Option C is incorrect. While this option does show a reduced compound with a new C–C bond, it is not the correct amount of carbon atoms.
Option D is incorrect. A Grignard reaction reduces a carbonyl to an alcohol by forming a new C–C bond. This compound is fully oxidized and is not reduced. Additionally, this compound does not contain the correct amount of carbon atoms.
2. Given the following product, which of following are possible Grignard reagents in this reaction? (Select all that apply.)
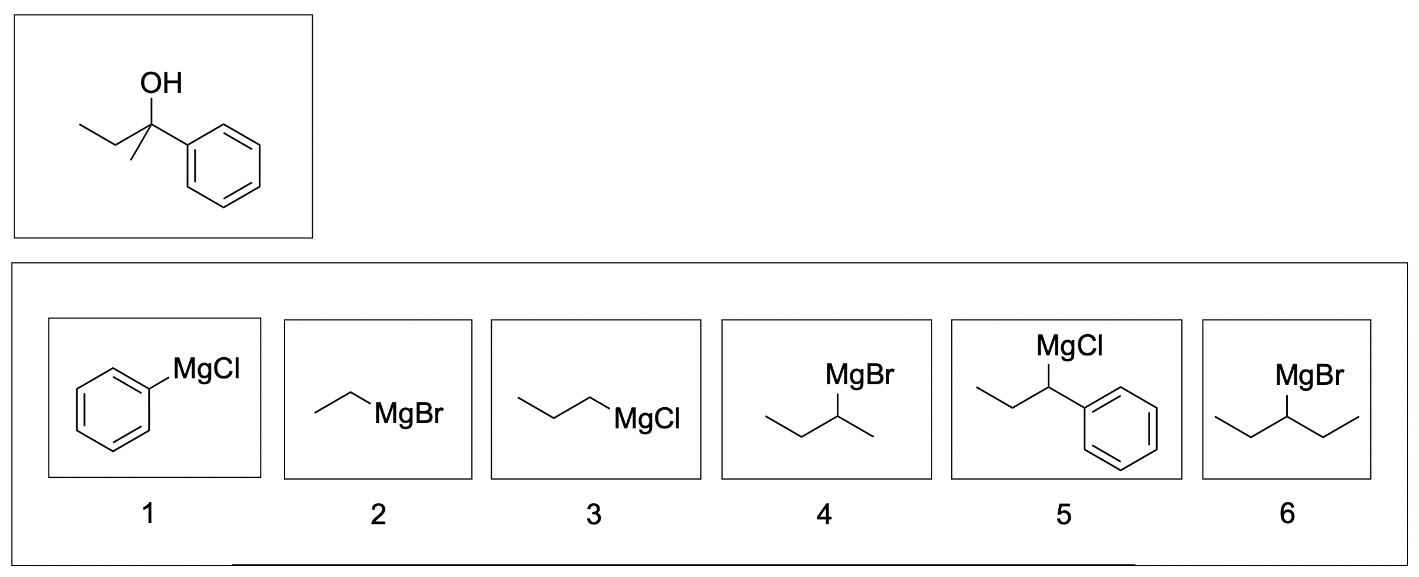
The correct answers to this question are Compound 1, 2 and 5. Grignard reactions form new C–C bonds, so it’s important to count all of the carbon atoms in the starting materials to ensure they add up to the products. Grignard reagents acts as nucleophiles and attack the electrophilic carbon center to form new C–C bonds. The addition of hydronium protonates the alkoxide to form an alcohol.
To identify potential Grignard reagents, start from the carbon atom bonded to the hydroxide (since this carbon atom was initially the electrophilic carbonyl), and treat each carbon group as a “branch”. For example, the compound can contain 3 carbon branches with 2, 1 and 6 carbon atoms respectively. The compound can also contain 2 branches with 3 and 6 carbon atoms respectively. Note that there are various combinations of “branches” that can be made, but the number of carbon atoms in the Grignard reagent must add up the number of carbon atoms in one of those branches since the Grignard reagent contains the exact number of carbon atoms being added to the compound. An example of 2 combinations of “branches” is shown in the photo below.
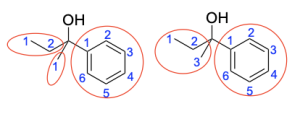
Option 1 is correct, as it is a possible Grignard reagent. It contains 6 carbon atoms in an aromatic structure like the product contains.
Option 2 is correct, as it is a possible Grignard reagent. It contains 2 carbon atoms in a linear structure like the product contains.
Option 3 is not a possible Grignard structure. It contains 3 carbon atoms in a linear structure, while the product does not contain any “branches” with 3 carbon atoms in a linear structure.
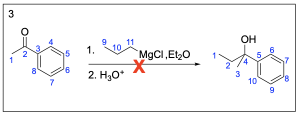
Option 4 is not a possible Grignard structure. The electrophile needed to obtain the end product with the given Grignard reagent is shown on the top–left corner, and it would lead to a product that contains an extra carbon atom. You would not be able to reduce the size of the electrophile because that would lead to a compound that contains 5 bonds to carbon (shown on the top–right corner), which is not possible.
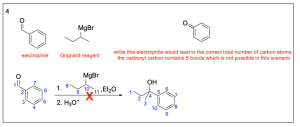
Option 5 is correct, as it is a possible Grignard reagent. It contains 9 carbon atoms (6 are in an aromatic structure while 3 are in a linear structure) like the product contains.
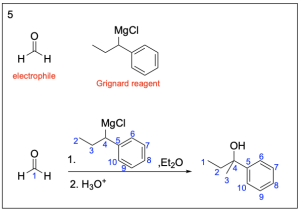
Option 6 is not a possible Grignard reagent. It contains 5 carbon atoms, and we know the final product must contain a 6–carbon benzene ring. That means that there would be at least 11 carbon atoms in the final product. But, the final product only has 10 carbon atoms, so this is not possible.
Chapter 3.4.3 – Comparing Sodium Borohydride Reductions and Grignard Reactions
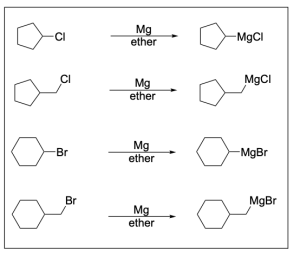
- Use group (1) to make Grignard reagent by reacting with Mg in diethyl ether, and then perform Grignard reaction with group (2). The product is then further reacted with KMnO4. Which of following molecules is not a possible final product?
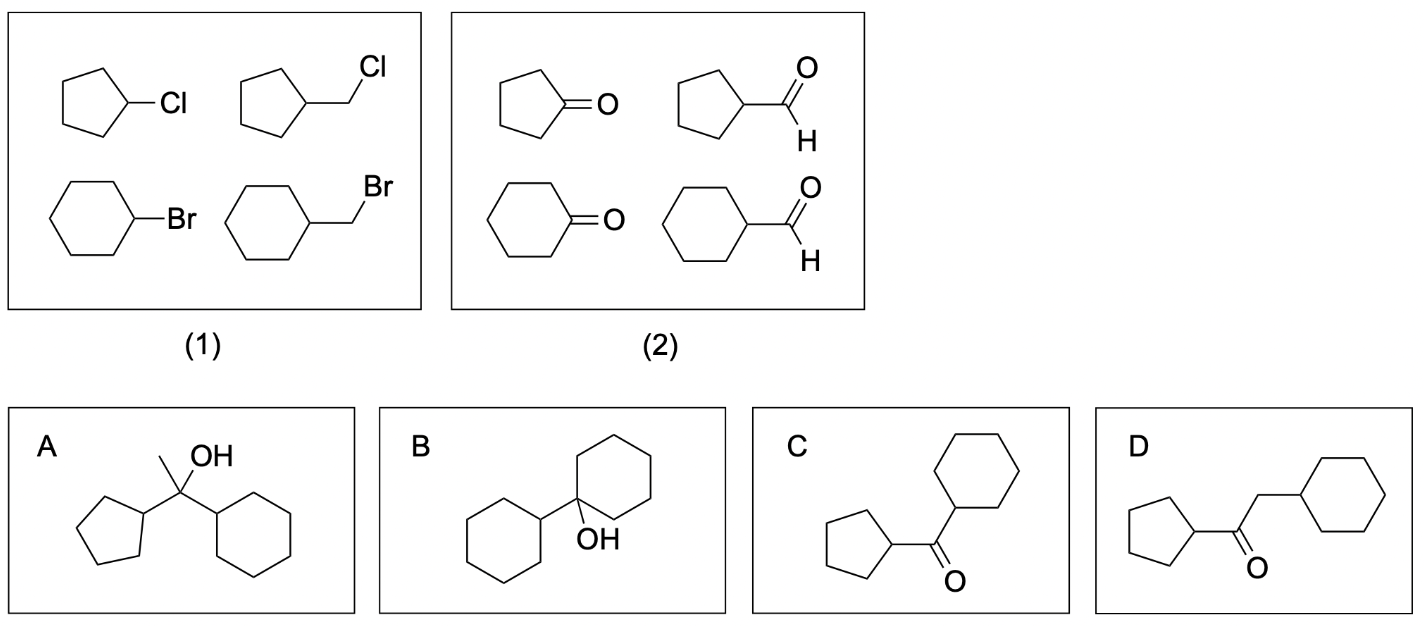
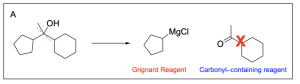
The correct answers to this question are Option B, C and D. Reacting an alkyl halide with magnesium in diethyl ether produces a Grignard reagent. By reacting all alkyl halides in group (1) with magnesium in diethyl ether, you will get the following Grignard reagents:
The question then tells us that the group 1 Grignard reagents are reacted with the carbonyl–containing compounds in group 2. The Grignard reagent is nucleophilic and attacks the electrophilic carbonyl forming new C–C bonds. The addition of hydronium will provide a proton source to form a reduced alcohol. Therefore, the products from step 2 would include an alcohol with a larger carbon chain.
The last piece of information the question tells us is that the product of the Grignard reaction is reacted with KMnO4. This is a strong oxidizing agent, and it can oxidize primary and secondary alcohols formed from the Grignard reaction in the previous step. Note that tertiary alcohols cannot be oxidized, so tertiary alcohols formed from the Grignard reactions will remain as is after being reacted with KMnO4.
The first thing you should always do with Grignard reactions is count the number of carbon atoms. Since there are 16 possible products formed when reacting groups 1 and 2, it’s best to first check if the number of carbon atoms in the starting materials add up to the number of carbon atoms in the product, as this can allow you to quickly eliminate some options. The maximum number of carbon atoms possible with the various reagents is 14, and all options have less than 14 carbon atoms, so the carbon count looks good.
The next thing you should do is “break up” the end product to try and decipher the reagents. You can do this by splitting the molecule in half where the alcohol/carbonyl is, because the Grignard reagents will react with the carbonyl–containing compound to form the alcohol.
Option A is incorrect. When “breaking up” the end product to decipher the reagents, the only possible Grignard reagent is the first, but the number of carbon atoms in the carbonyl–containing compound does not match any of the options listed.
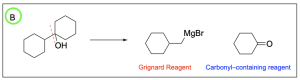
 Option B is correct. When “breaking up” the end product to decipher the reagents, both the Grignard reagent and the carbonyl–containing compound needed are listed as options in groups 1 and 2.
Option B is correct. When “breaking up” the end product to decipher the reagents, both the Grignard reagent and the carbonyl–containing compound needed are listed as options in groups 1 and 2.
 Option C is correct. When “breaking up” the end product to decipher the reagents, both the Grignard reagent and the carbonyl–containing compound needed are listed as options in groups 1 and 2, and there are 2 possible combinations that can lead to the end product.
Option C is correct. When “breaking up” the end product to decipher the reagents, both the Grignard reagent and the carbonyl–containing compound needed are listed as options in groups 1 and 2, and there are 2 possible combinations that can lead to the end product.
Option D is correct. When “breaking up” the end product to decipher the reagents, both the Grignard reagent and the carbonyl–containing compound needed are listed as options in groups 1 and 2.