5.2 – Solutions for Chapter 3 – Reactivity
Chapter 3.3 – Oxidation of Alcohols
1. Which of the following is the correct alcohol oxidation-reduction reaction chain?
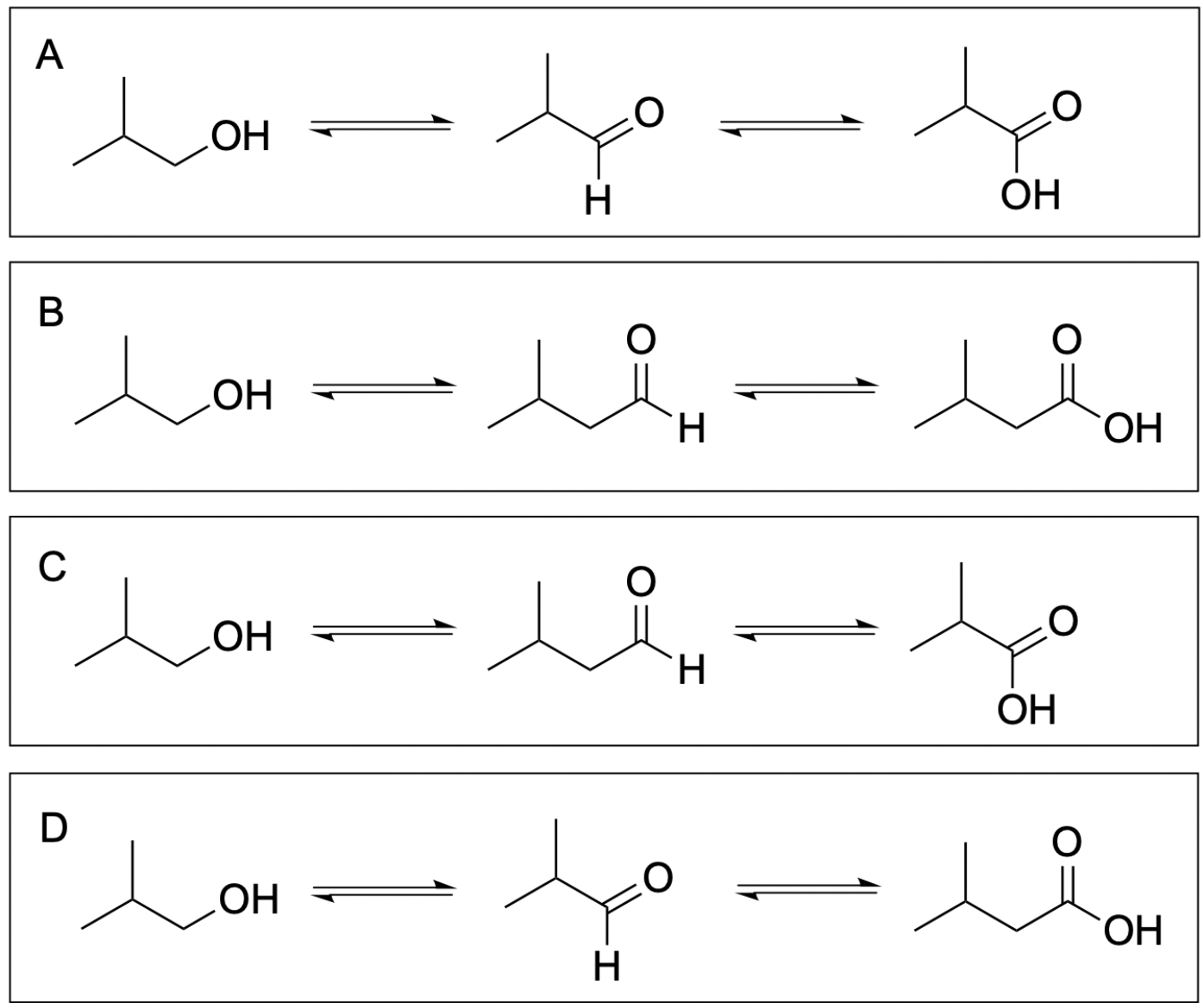
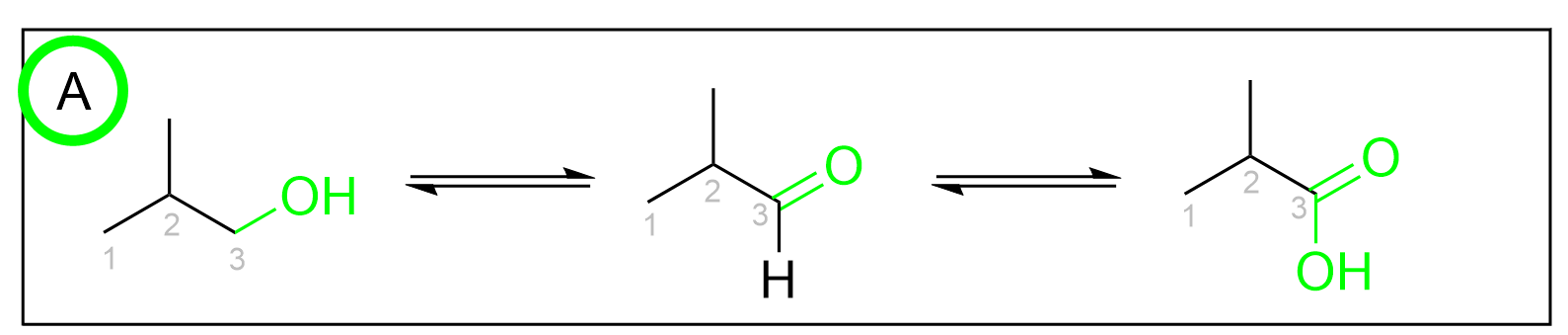
Option A is correct. In each oxidation event, the same carbon is becoming more oxidized, as it gains more bonds to oxygen. It starts as an alcohol with one C–O bond, becomes an aldehyde with two C–O bonds, and ends up as a carboxylic acid with three C–O bonds.
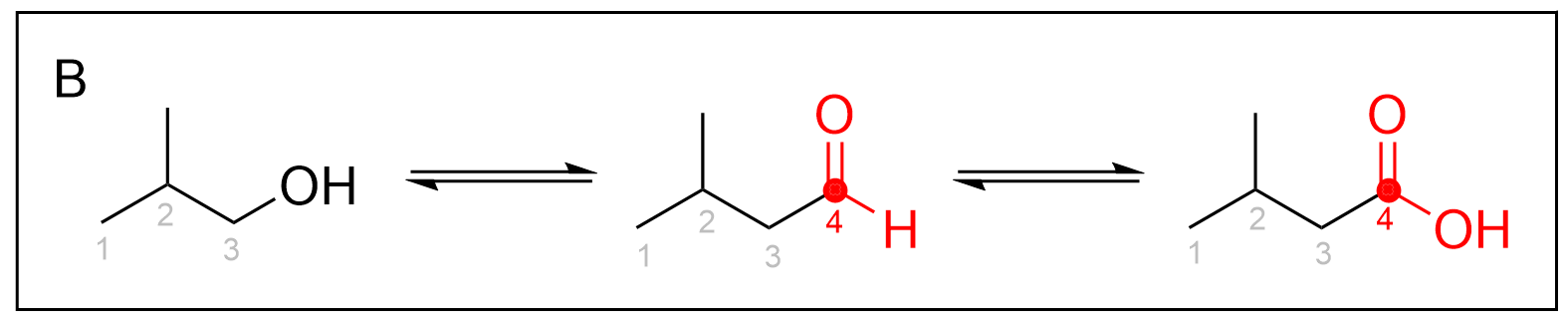
Option B is incorrect due to the number of carbon atoms in the aldehyde and carboxylic acid. Although at first glance the answer may also seem correct, what is important to notice is the number of carbons on each molecule. Oxidation reactions do not add any carbon atoms to a molecule. Taking a closer look at this reaction scheme, the starting molecule, propanol, has three carbons. However, the following aldehyde and carboxylic acids both contain four carbon atoms. Thus, this answer is incorrect as every molecule should only contain three carbon atoms.
Option C is incorrect due to the number of carbon atoms in the aldehyde. As the starting molecule, propanol, contains three carbons, each successive product should also contain only three carbons. Although the final product also contains three carbons, the middle aldehyde contains four carbons. Oxidation reactions do not add any carbon atoms to a molecule, and thus, this reaction scheme is impossible.
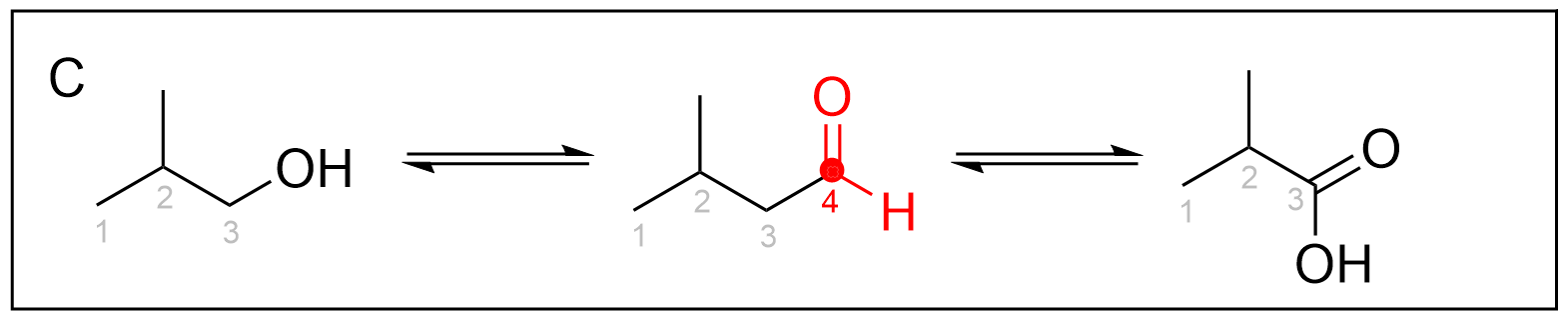
Option D is incorrect due to the number of carbon atoms in the carboxylic acid. As the starting molecule, propanol, contains three carbons, each successive product should also contain only three carbons. Although the aldehyde also contains three carbons, the final carboxylic acid contains four carbons. Oxidation reactions do not add any carbon atoms to a molecule, and thus, this reaction scheme is impossible.
2. Which statement about the oxidation of alcohols is false?
A. Primary alcohols can be oxidized to form either an aldehyde or a carboxylic acid.
B. Ketones and tertiary alcohols cannot be oxidized using KMnO4 or K2Cr2O7.
C. Primary alcohols can be oxidized using permanganate, dichromate, or PCC/ CH2Cl2.
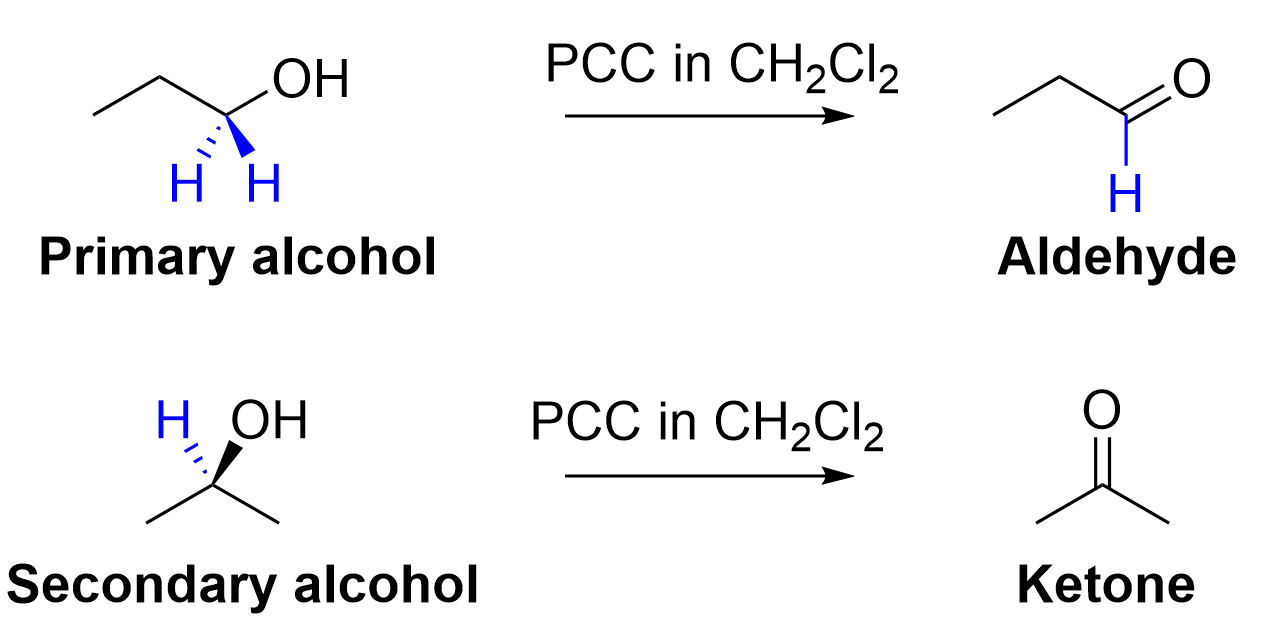
D. PCC/CH2Cl2 can be used to selectively oxidize secondary alcohols to aldehydes.
The correct answer is Option D. Although it is true that PCC in CH2Cl2 is a selective oxidizing agent, the wording of the statement makes it incorrect. Secondary alcohols cannot be oxidized into aldehydes and can only be oxidized into ketones. This is because of the number of carbons that the carbon on the C–O bond contains. The carbon in the C–O bond is only bonded to one hydrogen atom, as it is also bonded to two more carbon atoms. Thus, when oxidized with PCC in CH2Cl2, it loses that one hydrogen and will have no more C–H bonds. With no hydrogen, this classifies the functional group as a ketone rather than an aldehyde. It is only primary alcohols that can be oxidized into aldehydes. Refer to the diagram below for a more visual explanation of the oxidation of primary vs. secondary alcohols.