5.2 – Solutions for Chapter 3 – Reactivity
Chapter 3.2.1 – Hydrohalogenation of Alkenes
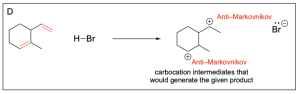
1. Which of the following are two possible intermediate states during this hydrohalogenation reaction?
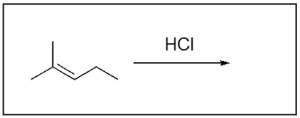
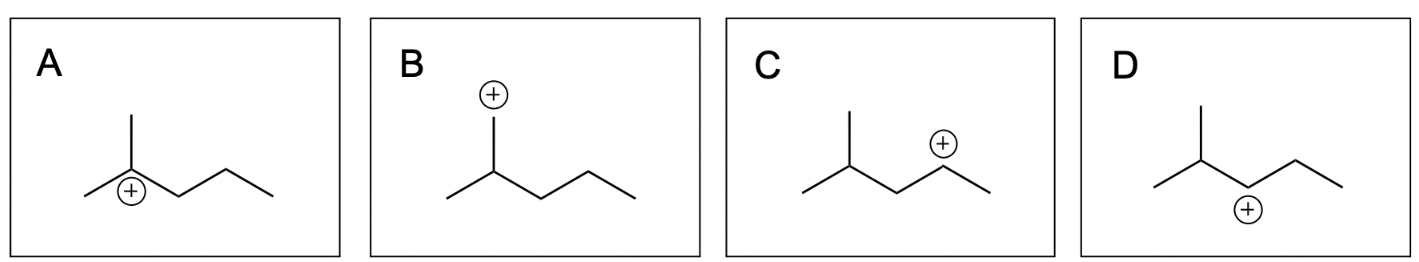
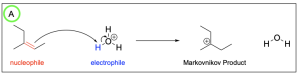
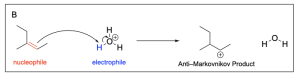
The correct answers to this question are Option A and Option D. Organic reactions involve an electron-rich source (the nucleophile) attacking an electron-deficient source (the electrophile). In hydrohalogenation, the alkene is nucleophilic and attacks the electrophilic hydrogen in the hydrogen halide in a mechanism similar to SN1, where there is a carbocation intermediate.
Option A is correct. This option illustrates the major product, or Markovnikov product, where the hydrogen atom is added to the less substituted carbon. This leaves a more stable carbocation intermediate that will then undergo nucleophilic attack by the chloride ion.
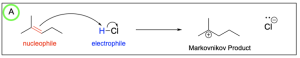
Option B is incorrect. There is not a nucleophilic source (such as an alkene) on the carbon atom indicated. There must be a nucleophilic source (second reaction scheme in the photo below) to attack the electrophilic hydrogen atom in order to undergo a hydrohalogenation reaction.
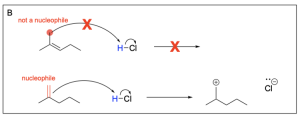
Option C is incorrect. There is not a nucleophilic source (such as an alkene) on the carbon atom indicated. There must be a nucleophilic source (second reaction scheme in the photo below) to attack the electrophilic hydrogen atom in order to undergo a hydrohalogenation reaction.
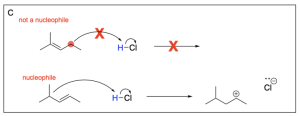
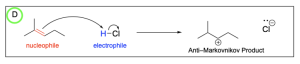
Option D is also correct. This option illustrates the minor product, or Anti–Markovnikov product, where the hydrogen atom is added to the more substituted carbon, leaving a less stable carbocation intermediate that will then undergo nucleophilic attack by the chloride ion.
2. Which of following is the most likely product of hydrohalogenation?
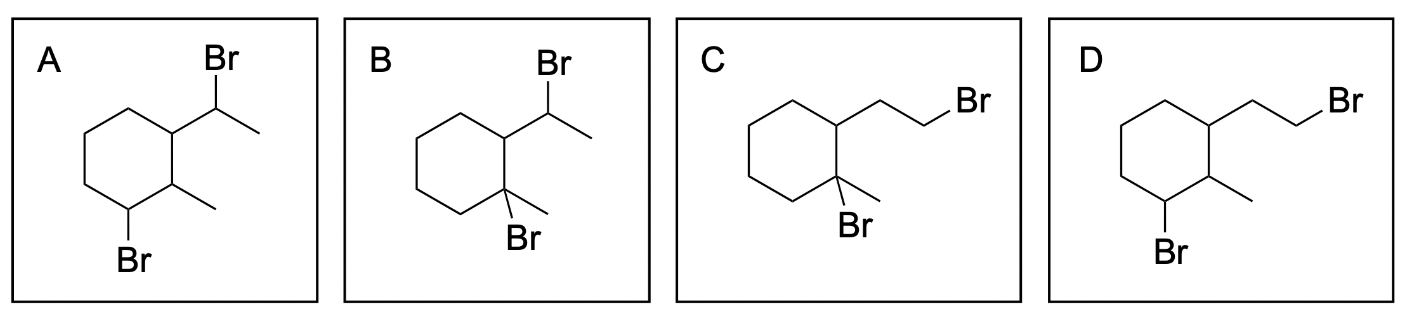
The correct answer to this question is Option B. Hydrohalogenation reactions involve a nucleophilic alkene attacking an electrophilic hydrogen halide where the hydrogen and halogen are added to a carbon atom in the alkene in a mechanism similar to SN1, where there is a carbocation intermediate. In unsymmetrical alkenes, there are 2 atoms the hydrogen and halide can be added to: the more substituted and less substituted carbon atom. The more stable carbocation intermediate is at the more substituted carbon where the nucleophilic halogen can attack. This will generate the more favourable Markovnikov product, which will form more readily than the Anti–Markovnikov product.
Option A is incorrect. This can be a product of hydrohalogenation, but it is not the most favourable out of the options listed. While the alkene on the right forms the more substituted and favourable Markovnikov product, the alkene on the left forms the less substituted Anti–Markovnikov product. This is less favourable and will not form as readily.
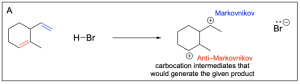
Option B is correct. This is the most favourable product of hydrohalogenation out of the options listed. Both alkenes form the more substituted and favourable Markovnikov product. Both are favourable and will readily form.
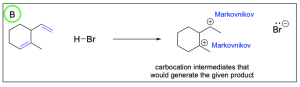
Option C is incorrect. This can be a product of hydrohalogenation, but it is not the most favourable out of the options listed. While the alkene on the left forms the more substituted and favourable Markovnikov product, the alkene on the right forms the less substituted Anti–Markovnikov product. This is less favourable and will not form as readily.
Option D is incorrect. This can be a product of hydrohalogenation, but it is the least favourable out of the options listed. Both alkenes form the less substituted and less favourable Anti–Markovnikov product. Both are not favourable and will not readily form.
Chapter 3.2.2 – Hydration of Alkenes
1. Which of the following are intermediates of alkene hydration?
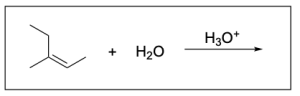
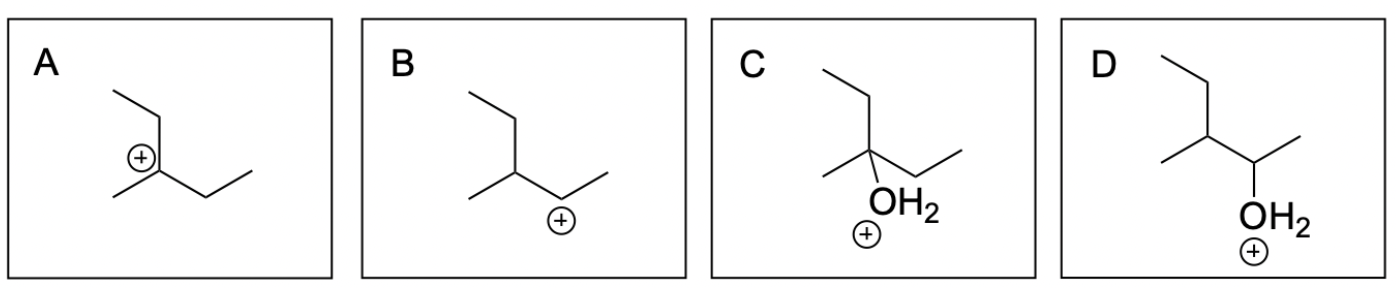
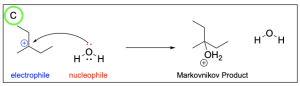
The correct answer to this question is Option A. Hydration reactions involve the nucleophilic alkene attacking hydronium, where the hydrogen and hydroxide are added to a carbon atom in the alkene in a mechanism similar to SN1, where there is a carbocation intermediate. A key difference between the mechanism in a hydration reaction vs. an SN1 reaction is that hydration reactions contain an extra deprotonation step since water is neutral. In unsymmetrical alkenes, there are 2 atoms the hydrogen and hydroxide can be added to: the more substituted and less substituted carbon atom. The more stable carbocation intermediate is at the more substituted carbon where the nucleophilic water can attack. This will generate the more favourable Markovnikov product, which will form more readily than the Anti–Markovnikov product.
Option A is correct. This is one of the most favourable hydration intermediates out of the options listed. The nucleophilic alkene attacks the electrophilic hydronium generating a carbocation intermediate on the most substituted carbon atom. This is more favourable and will occur more readily.
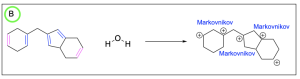
Option B is incorrect. This is not the most favourable hydration intermediate out of the options listed. The nucleophilic alkene attacks the electrophilic hydronium generating a carbocation intermediate on the least substituted carbon atom. This is less favourable and will not readily occur.
Option C is correct. This is one of the most favourable hydration intermediates out of the options listed. The neutral water will attack the carbocation intermediate generating an oxonium cation intermediate. Due to the favourable more substituted carbocation intermediate, this intermediate will form more readily.
Option D is incorrect. This is not the most favourable hydration intermediate out of the options listed. The neutral water will attack the carbocation intermediate generating an oxonium cation intermediate. Due to the unfavourable less substituted carbocation intermediate, this intermediate will not readily form.
2. Which of following is a possible product of the alkene hydration reaction?
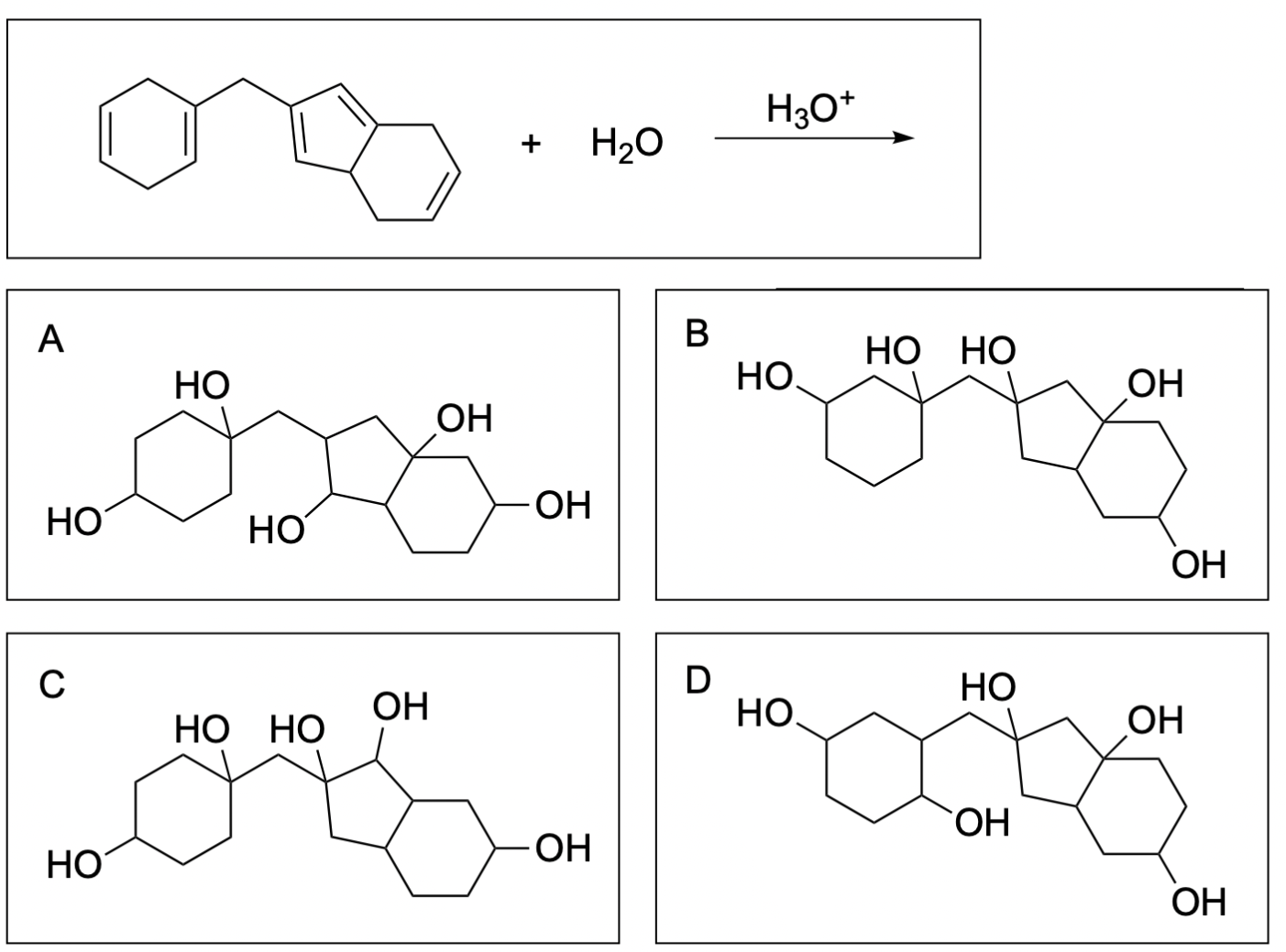
The correct answer to this question is Option B. Hydration reactions involve the nucleophilic alkene attacking hydronium, where the hydrogen and hydroxide are added to a carbon atom in the alkene in a mechanism similar to SN1, where there is a carbocation intermediate. In symmetric alkenes, there is no preference in which carbon the water is added to since the same carbocation intermediate is generated. In unsymmetrical alkenes, there are 2 atoms the hydrogen and hydroxide can be added to: the more substituted and less substituted carbon atom. The more stable carbocation intermediate is at the more substituted carbon where the nucleophilic halogen can attack. This will generate the more favourable Markovnikov product, which will form more readily than the Anti–Markovnikov product.
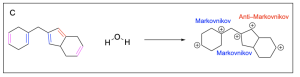
Option A is incorrect. This can be a product of hydration, but it is not the most favourable out of the options listed. The alkenes highlighted in pink are symmetric, so water is equally likely to attack either carbon atom. The alkenes highlighted in blue generated a favourable and more substituted carbocation intermediate (Markovnikov product), which will form more readily. The alkene highlighted in red generated an unfavourable carbocation intermediate (Anti–Markovnikov product), which will not readily form.
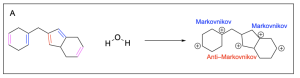
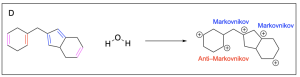
Option B is correct. This is the most favourable out of the options listed. The alkenes highlighted in pink are symmetric, so water is equally likely to attack either carbon atom. The alkenes highlighted in blue generated a favourable and more substituted carbocation intermediate (Markovnikov product), which will form more readily.
Option C is incorrect. This can be a product of hydration, but it is not the most favourable out of the options listed. The alkenes highlighted in pink are symmetric, so water is equally likely to attack either carbon atom. The alkenes highlighted in blue generated a favourable and more substituted carbocation intermediate (Markovnikov product), which will form more readily. The alkene highlighted in red generated an unfavourable carbocation intermediate (Anti–Markovnikov product), which will not readily form.
Option D is incorrect. This can be a product of hydration, but it is not the most favourable out of the options listed. The alkenes highlighted in pink are symmetric, so water is equally likely to attack either carbon atom. The alkenes highlighted in blue generated a favourable and more substituted carbocation intermediate (Markovnikov product), which will form more readily. The alkene highlighted in red generated an unfavourable carbocation intermediate (Anti–Markovnikov product), which will not readily form.
Chapter 3.2.3 – Hydrogenation of Alkenes
1. Which of the following is the product of the alkyne hydrogenation?
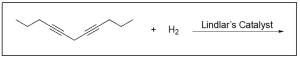
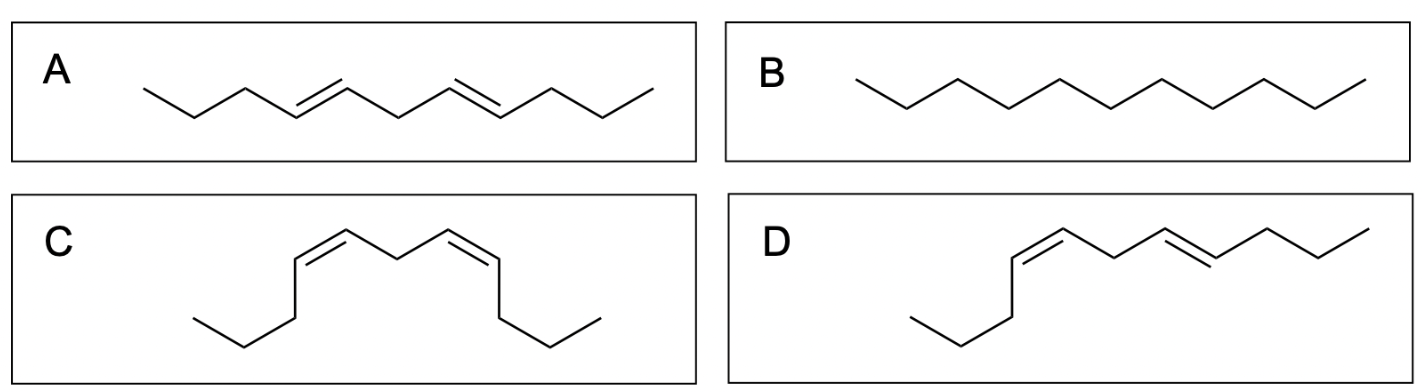
The correct answer to this question is Option C. Hydrogenation reactions involve the addition of hydrogen to an alkene or alkyne due to the presence of a metal catalyst. Lindlar’s catalyst is a poisoned catalyst, so it does not fully reduce the alkyne to an alkane. It will instead reduce an alkyne to a cis-alkene.
Option A is incorrect. While Option A does correctly show partial reduction to an alkene, it does not correctly show a cis-alkene as a product, and instead shows a trans-alkene. Note that it is possible to reduce an alkyne to a trans-alkene, but it is not under these conditions, and you will learn more about this is your second year organic chemistry class.

Option B is incorrect. Lindlar’s catalyst does not fully reduce an alkyne to an alkane. To do this, you would need excess hydrogen gas in the presence of Pd/C catalyst (shown in the second reaction scheme below).
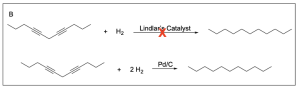
Option C is correct. Lindlar’s catalyst will reduce an alkyne to a cis–alkene.

Option D is incorrect. While the alkene highlighted in blue has been correctly reduced to a cis–alkene, the alkene highlighted in red has not been correctly reduced. It should have been reduced to a cis, not a trans–alkene.

Chapter 3.2.4. – Halogenation of Alkenes
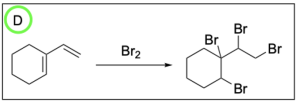
1. Which of the following is the product of halogenation?
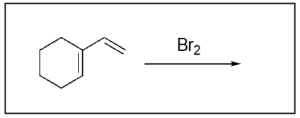
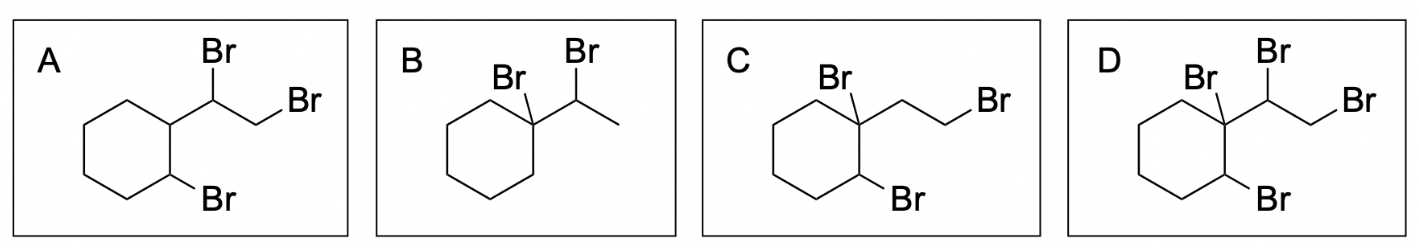
The correct answer to this question is Option D. Halogenation reactions involve the addition of a halogen across an alkene. A key difference between halogenation and hydrohalogenation is that there is no Markovnikov addition in halogenation reactions since the same atom is being added across the double bond.
Option A is incorrect. In halogenation, the halogen will be added to both alkene carbons. The option illustrates this for the alkene on the right, but does not show this for the alkene on the left.
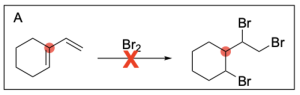
Option B is incorrect. In halogenation, the halogen will be added to both alkene carbons. The option does not illustrate this for either alkene.
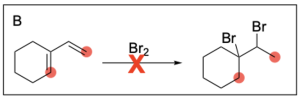
Option C is incorrect. In halogenation, the halogen will be added to both alkene carbons. The option illustrates this for the alkene on the left, but does not show this for the alkene on the right.
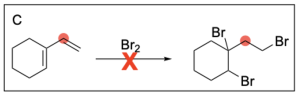
Option D is correct. In halogenation, the halogen will be added to both alkene carbons. This option illustrates this for both alkenes.