5.1 – Solutions for Chapter 2 – Organic Structure and Bonding
Chapter 2.5.1 – Alkene Structure
1. Which is the correct ranking of the melting point of the molecules from lowest to highest?
A. 4, 3, 2, 1
B. 4, 2, 3, 1
C. 2, 3, 1, 4
D. 3, 2, 1, 4
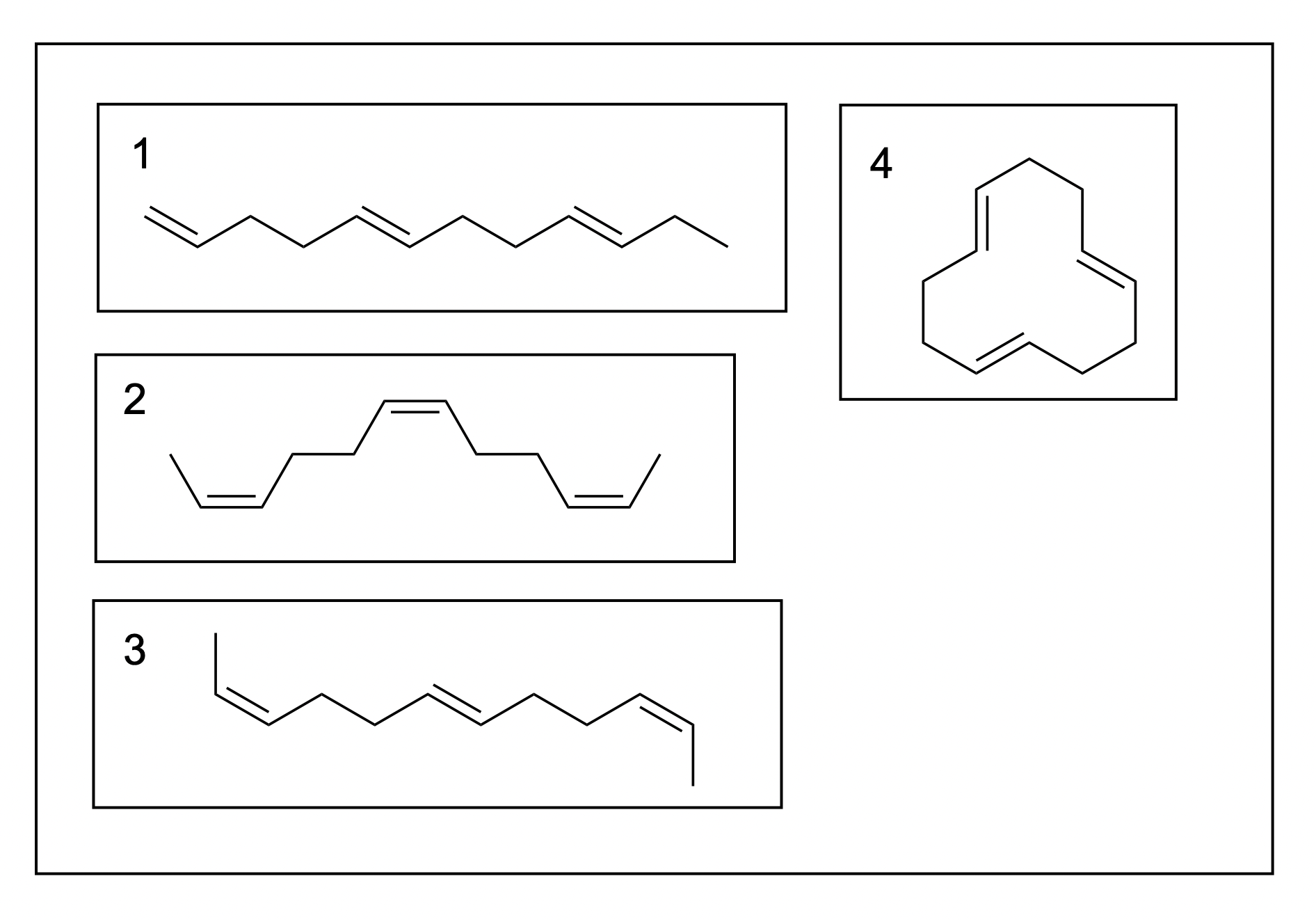
The correct answer to this question is Option B. Recall that melting point is correlated with the number of intermolecular forces, such as hydrogen bonding, Van der Waals and hydrophobic interaction. The more stacking that can occur between different molecules, the higher the melting point. Thus, trans alkenes have a significantly higher melting point as they can stack more efficiently, whereas cis alkenes introduce kinks that disrupt stacking.
As a ring, molecule 4 has the most trouble stacking on top of one another, giving it the lowest melting point. It does not matter whether the double bonds are trans or cis – all that matters is that it is cyclic. This eliminates two options, only leaving Option A and B as potential answers.
Molecule 1, as the only cis alkene on the list, automatically has the highest melting point due to its stacking efficiency.
Between molecule 2 and 3, it can be difficult to determine which would result in a greater number of intermolecular interactions. However, molecule 2 has three cis double bonds, whereas molecule 3 has only two. As it is the cis bonds that impede bonding, molecule 2 would have a lower melting point as it has more cis bonds.
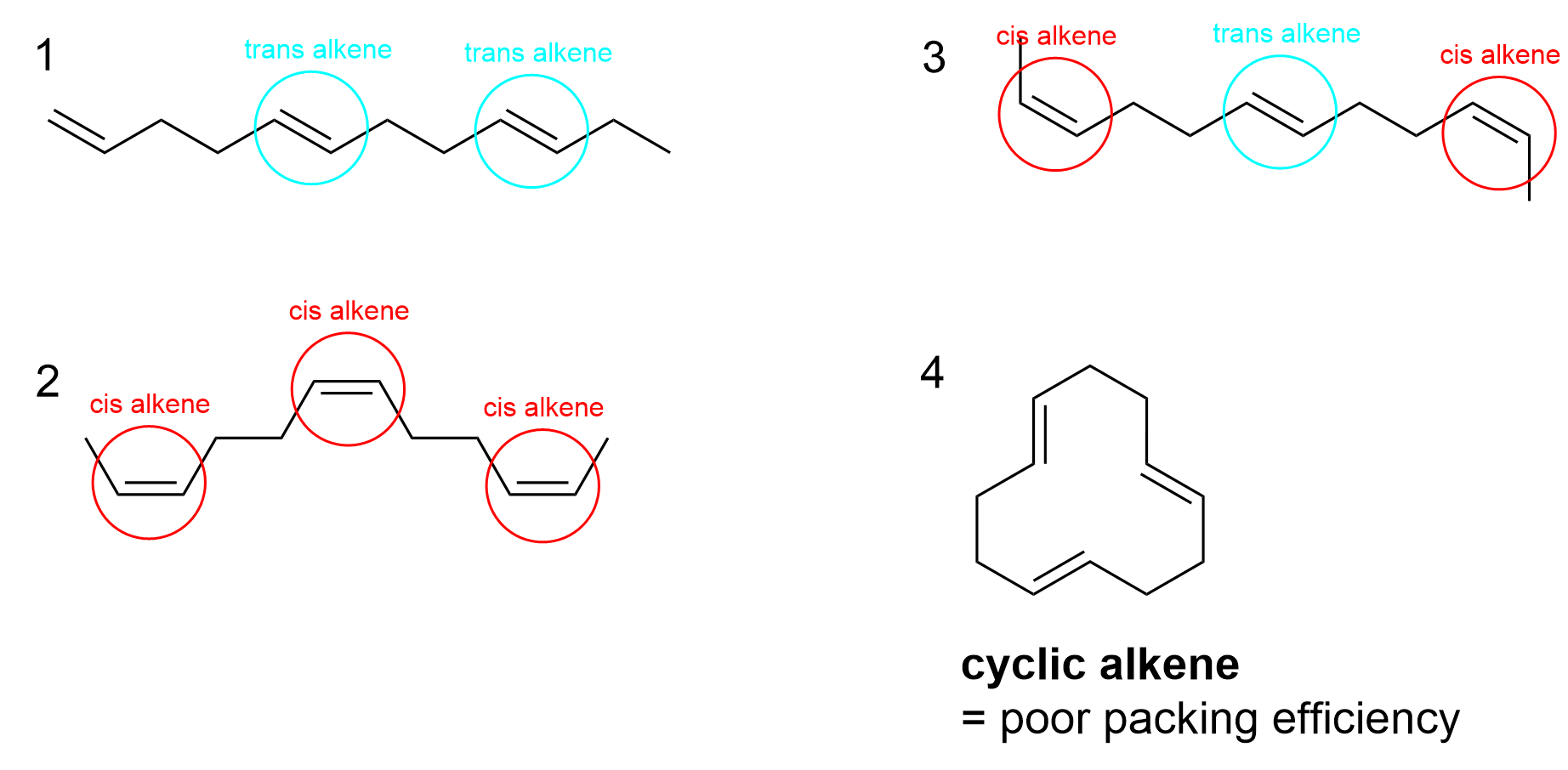
Therefore, the overall order from lowest to highest melting point would be: 4, 2, 3, 1 (Option B).
Chapter 2.5.2 – Alkene Stereochemistry & Nomenclature
1. Which of following molecules can use E/Z designation for nomenclature?
A. 1, 2
B. 2, 3
C. 3, 4
D. 2, 4
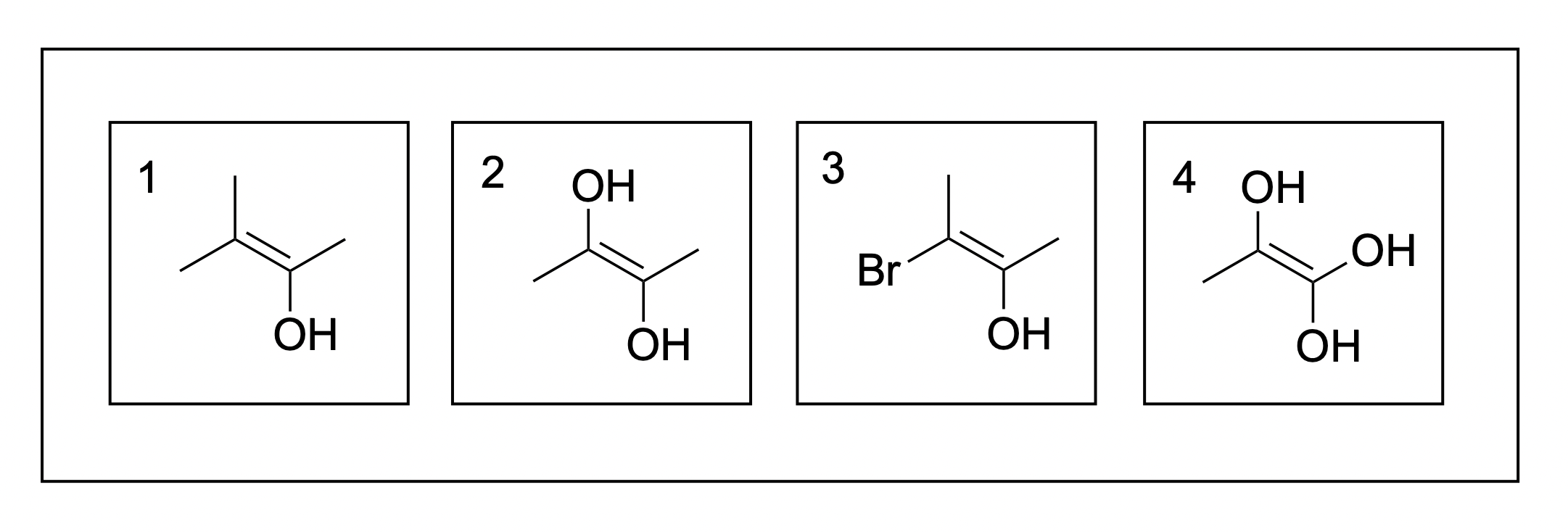
The correct answer to this question is Option B. The easiest way to determine this is to identify the substituents on each side of the C=C double bond and determine if it is possible to assign priority to the substituents. If it is not possible, then an E/Z designation cannot be used.
For example, in molecule 1, it is not possible to give an E/Z designation. The left side of the alkene contains two methyl groups, making it impossible to give one priority over the other. As assigning priority is crucial for naming, this means this compound cannot be named via E/Z designation. Molecule 4 faces the same problem as both substituents on the right side of the alkene are hydroxyl. Once again, it is not possible to assign priority.
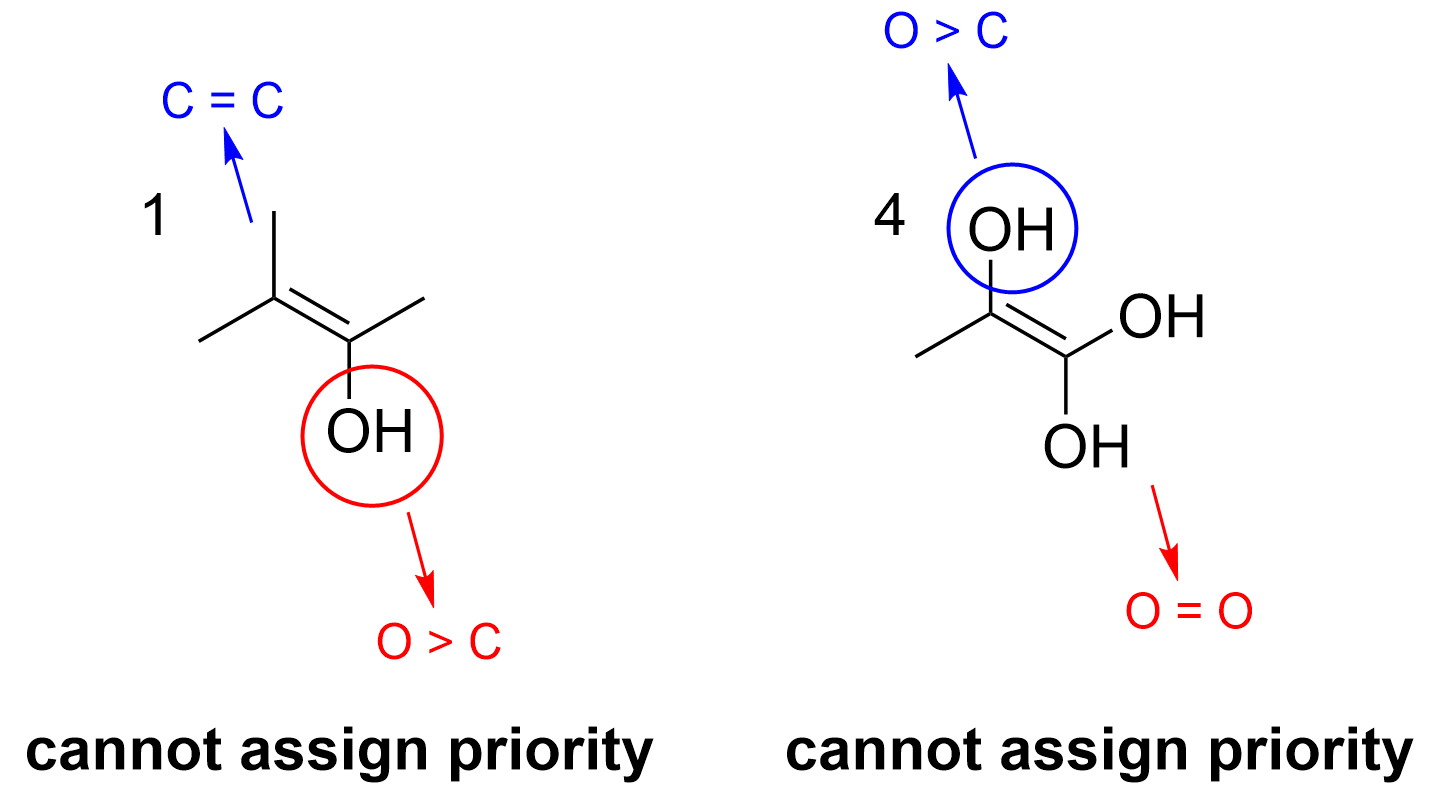
This issue is not faced in molecule 2 or 3, where it is possible to assign priority between the substituents on each side of the alkene. For example, in molecule 2, –OH takes priority over the methyl group, giving it an E arrangement. In molecule 3, the Br and OH take priority respectively, giving it a Z notation.
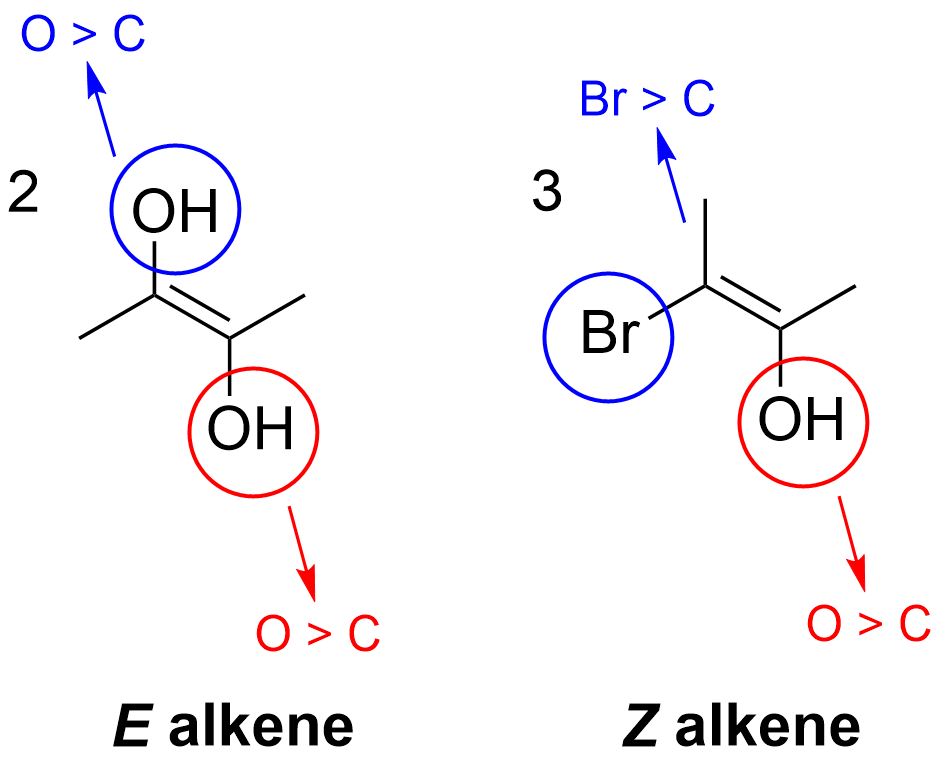
Thus, the correct answer is Option B (molecules 2 and 3).
2. Which of the following is the correct name for the molecule?
A. (2Z,5E)-4-ethenyl-3-((E)-3-methylhex-3-en-4-yl)hepta-2,5-dien-1-ol
B. (2Z,5E)-4-ethenyl-3-((Z)-3-methylhex-3-en-4-yl)hepta-2,5-dien-1-ol
C. (2Z,4E)-4-ethyl-3-((E)-hexa-1,4-dien-3-yl)-5-methylhepta-2,4-dien-1-ol
D. (2E,4Z)-4-ethyl-3-((E)-hexa-1,4-dien-3-yl)-5-methylhepta-2,4-dien-1-ol
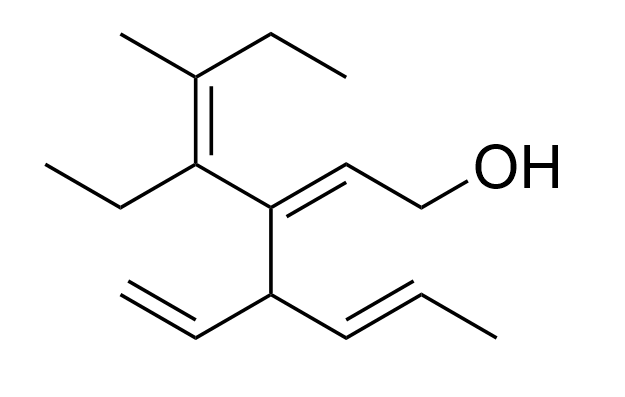
The correct answer is Option D.
Although this may look like a challenging question, this can be easily solved by only looking at specific parts of the full IUPAC name, specifically the E/Z notation. This can be done by (1) determining the parent chain and (2) looking at the E/Z designation for each double bond.
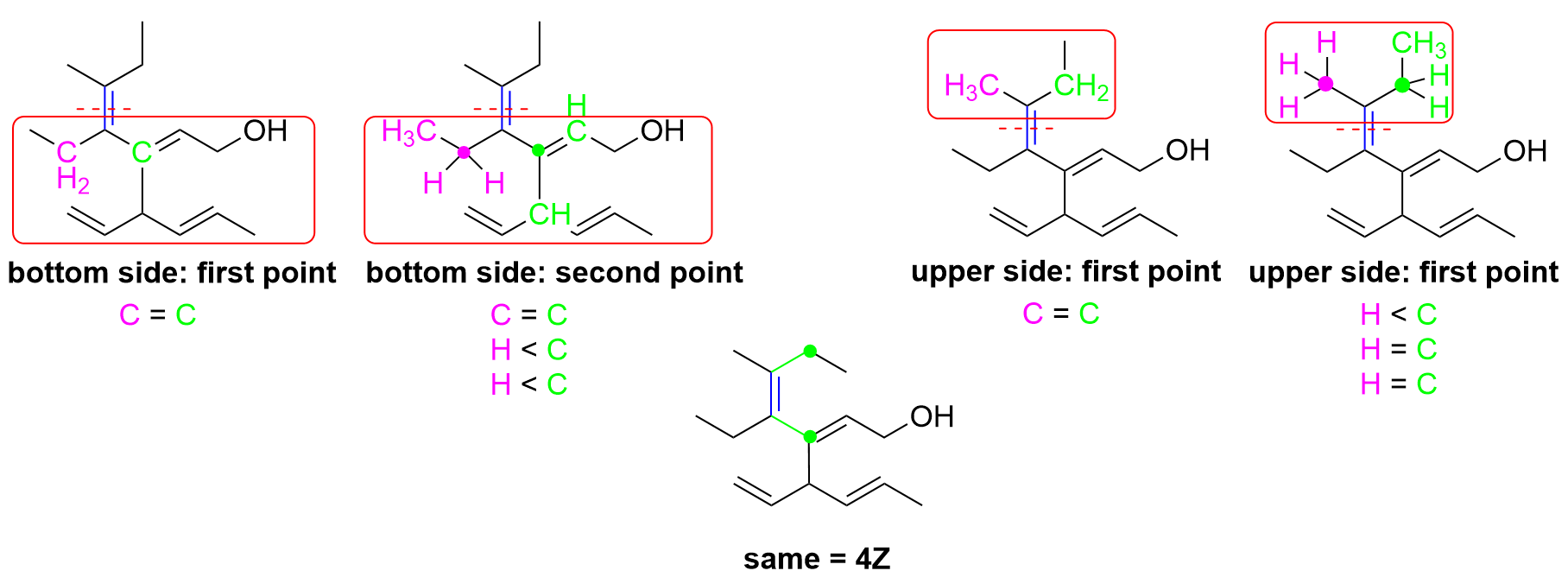
Determining the parent chain will help us know what positions the double bonds are in. As this compound only contains one functional group (the hydroxyl), we know the parent chain must contain this group. Furthermore, it should be the longest carbon chain encompassing the greatest number of double bonds. There are currently two options for the longest carbon chain – either the first one (in green) which goes upwards, or the second one (in blue) which goes downwards (pictured below)
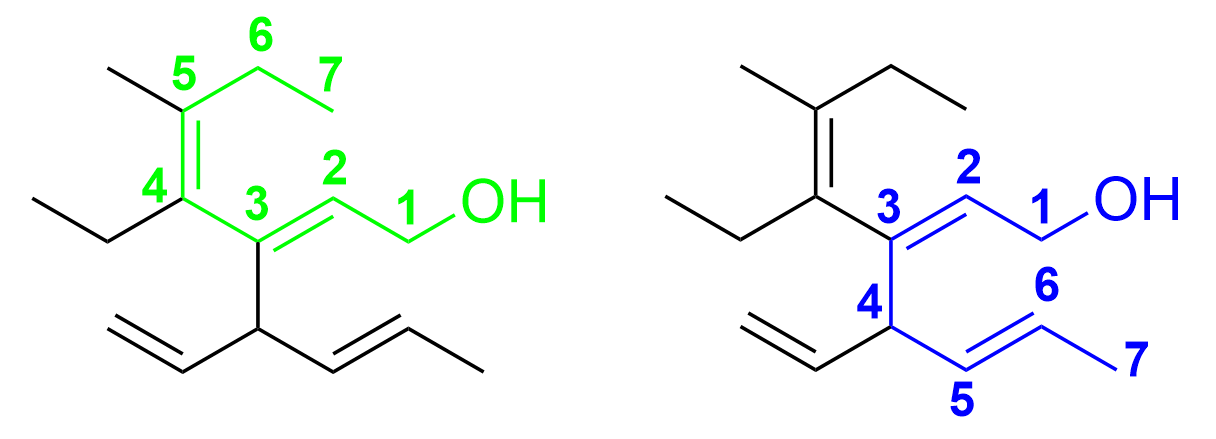
As both chains contain 7 carbons and 2 alkenes, it is equivalent in this sense. The next rule we must follow then, is to choose the chain where the positions of the functional groups are lower. As the carbon bonded to the hydroxyl group is designated as position 1, the green chain has the alkenes located at positions 2 and 4. In contrast, the blue chain has the alkenes at positions 2 and 5. As the double bonds appear on the green chain at lower position numbers, this is considered the parent chain of the molecule.
We can then identify the positions of each double bond and designate them as E or Z. There is a double bond found at position 2 and 4 on the parent chain. Just from determining the E/Z notations of these double bonds will give us the answer through process of elimination.
Let’s determine the E/Z notation at position 2 first. On the right side, it is easy to determine that priority is given to the carbon chain, as C has an atomic number of 6, whereas the other substituent is H, which has an atomic number of 1. The other side is slightly more difficult because both substituents attached to the double bond are carbon groups (green dots). However, the top carbon is counted to be bonded to three carbons (magenta dots) as it contains one C=C double bond and one C–C single bond. In contrast, the bottom carbon is bonded to only two carbons (magenta dots) and also contains one C–H bond. As C has a higher priority over H, the top carbon substituent takes priority on the left side. Thus, as this double bond has the higher priority groups pointing in opposite directions, this is an E alkene, and would be written as 2E in the IUPAC name.
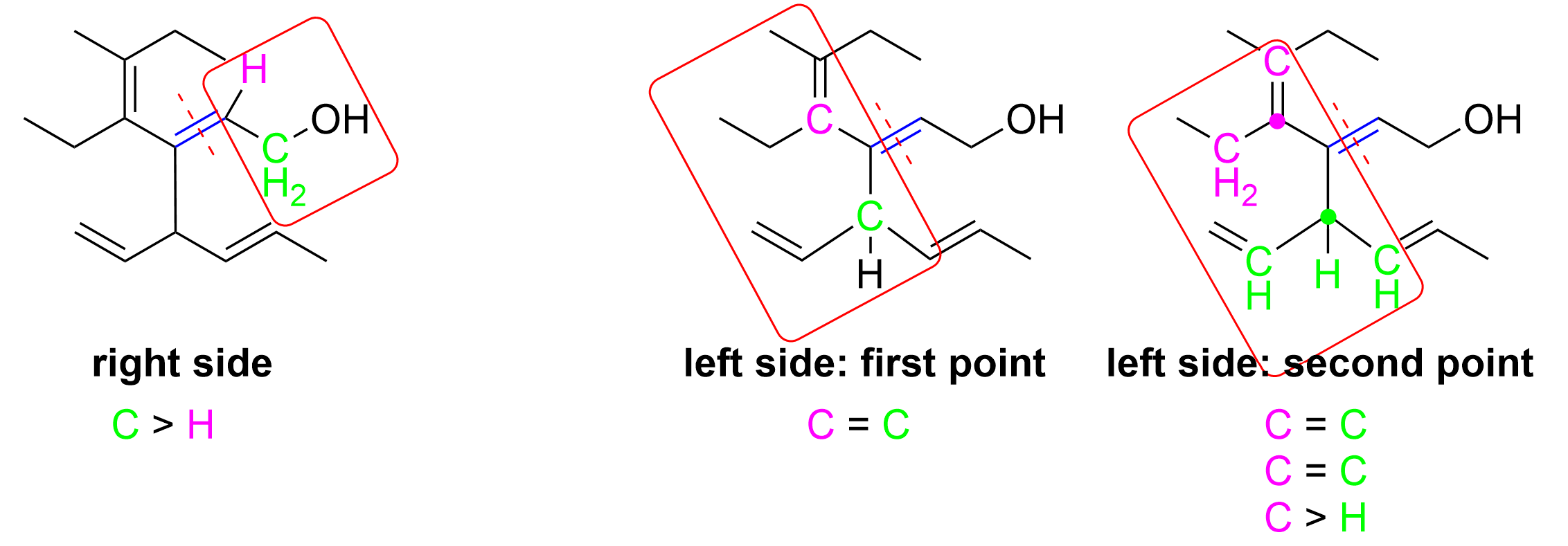
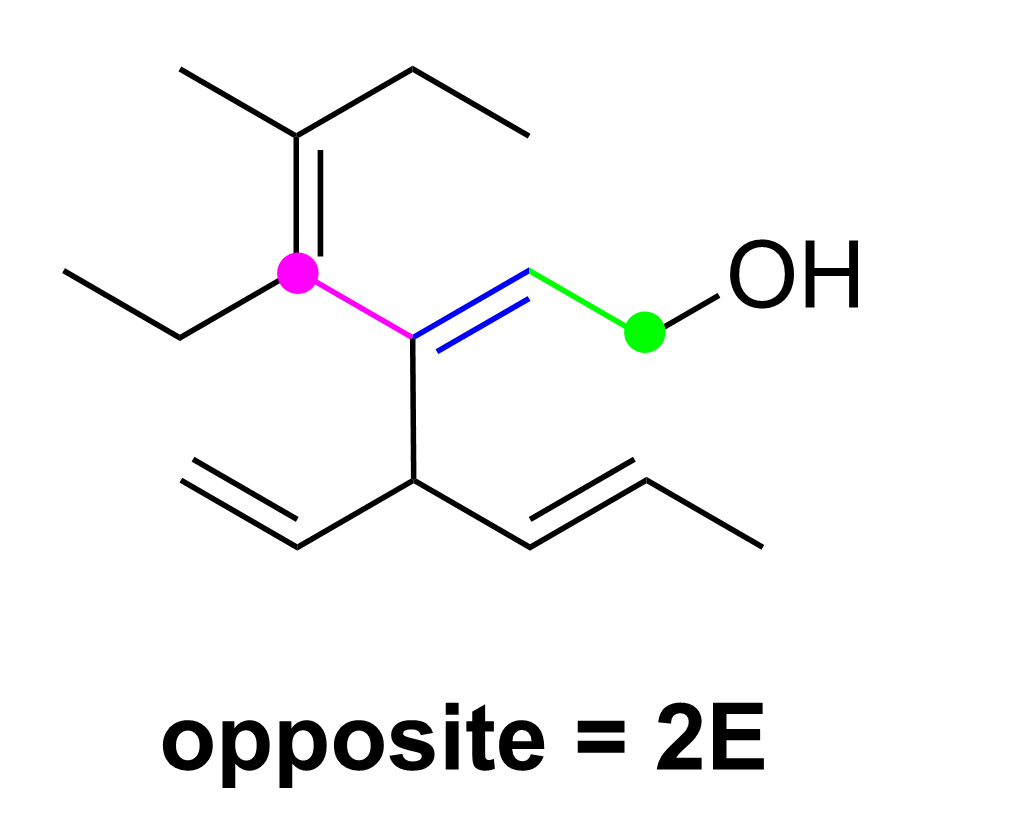
As only Option D contains 2E in the name, these two steps will quickly give us the correct answer already. For your own reference, the diagram below also explains the steps to determine the Z designation for the alkene at position 4.