5.1 – Solutions for Chapter 2 – Organic Structure and Bonding
Chapter 2.4 – IUPAC Nomenclature
1. Which is the correct name for the following molecule?
A. 3-hydroxyhept-6-enal
B. 5-hydroxyhepten-7-al
C. 3-hydroxyhept-7-enal
D. 5-hydroxyhepten-6-al
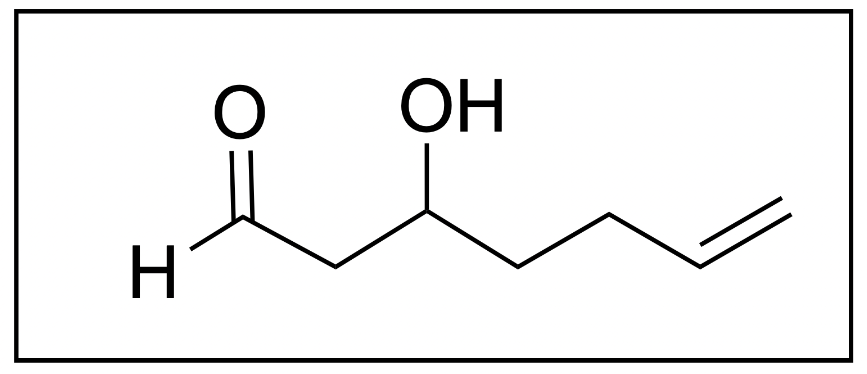
The correct answer is Option A. When naming organic molecules, the first thing you should do is identify the functional groups present to determine priority.
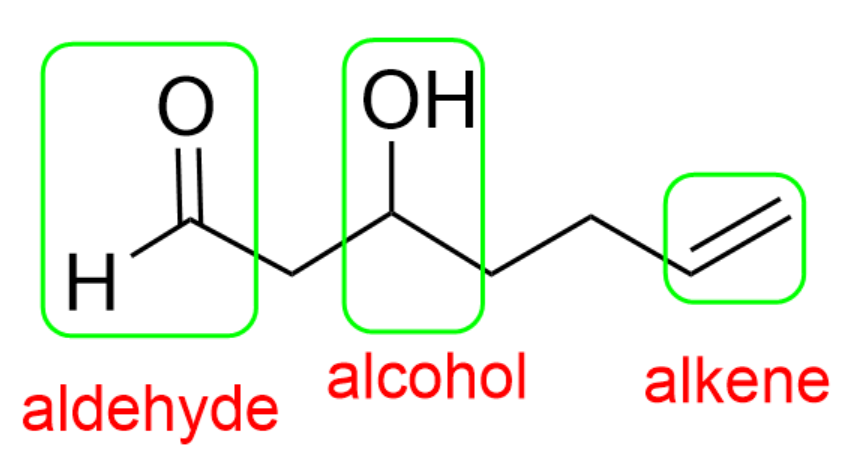
In this molecule, there is an aldehyde, alcohol and alkene present. The aldehyde is the highest priority group, so the final suffix is “-al”, while the alcohol will be treated as a substituent with the “hydroxy-“ prefix.
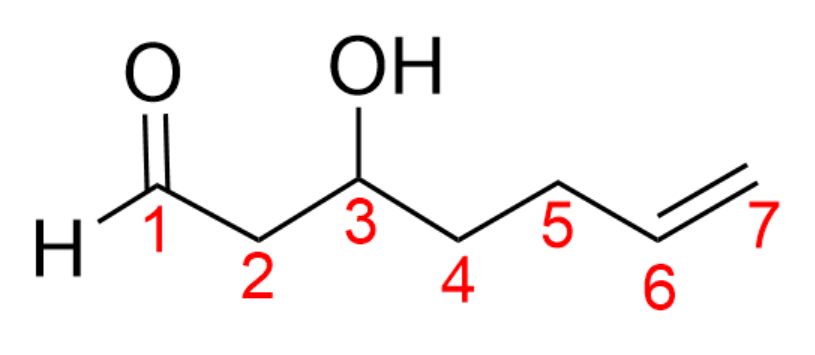
The next thing you should do is count the number of carbon atoms in the chain and decide which carbon atom is carbon 1. There are 7 carbon atoms present, so the prefix will be “hept”, and the number should begin by the highest priority group (the carbonyl is carbon 1).
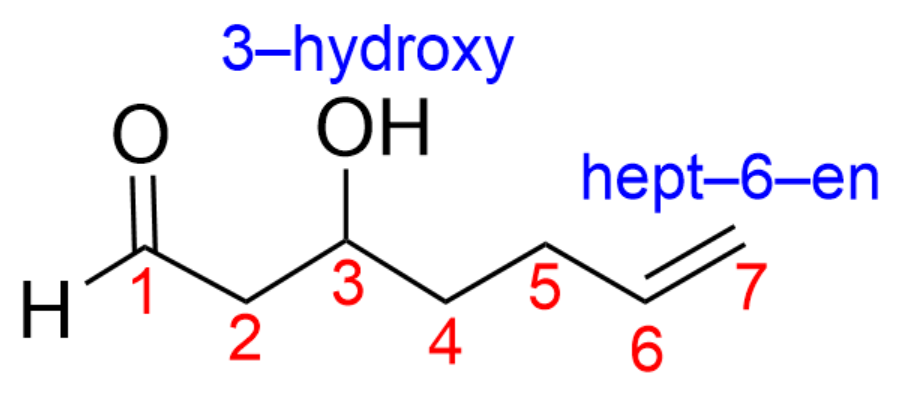
The hydroxy group is therefore at position 3 (3-hydroxy), while the alkene is at position 6 (hept-6-en).
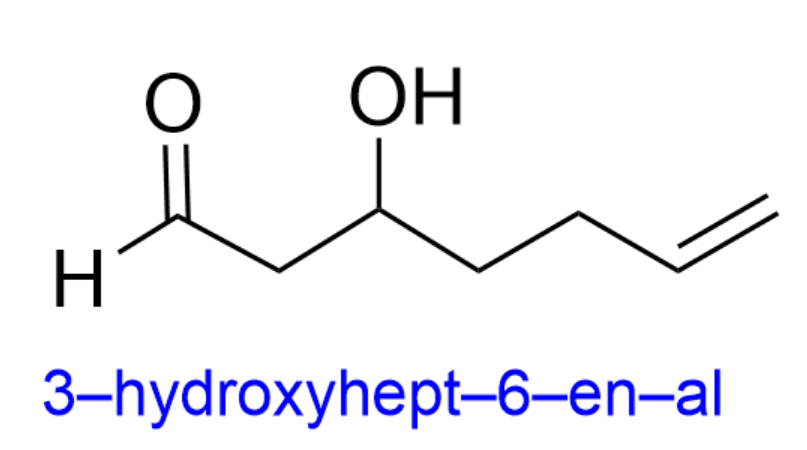
By putting all of this information together, we get the final name, 3-hydroxyhept-6-en-al. Therefore, the correct answer is Option A.
2. Which is the correct name for the following molecule?
A. 9-fluoro-3-iodo-3-methylnonane
B. 9-fluoro-3-methyl-3-iodononane
C. 1-fluoro-7-iodo-7-methylnonane
D. 7-iodo-1-fluoro-7-methylnonane
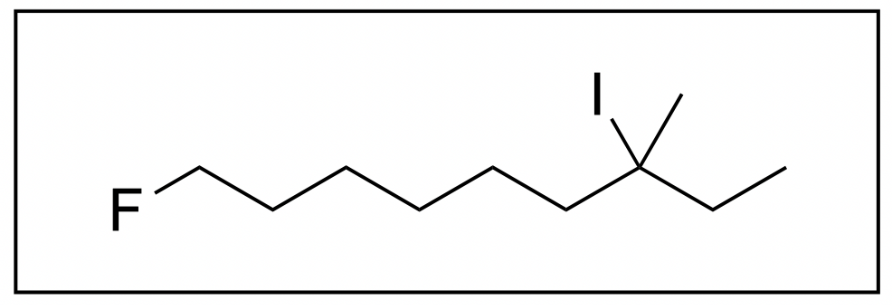
The correct answer is Option C. When naming organic molecules, the first thing you should do is identify the functional groups present to determine priority.
This organic molecule only contains an alkane functional group, so the final suffix will be “-ane”.
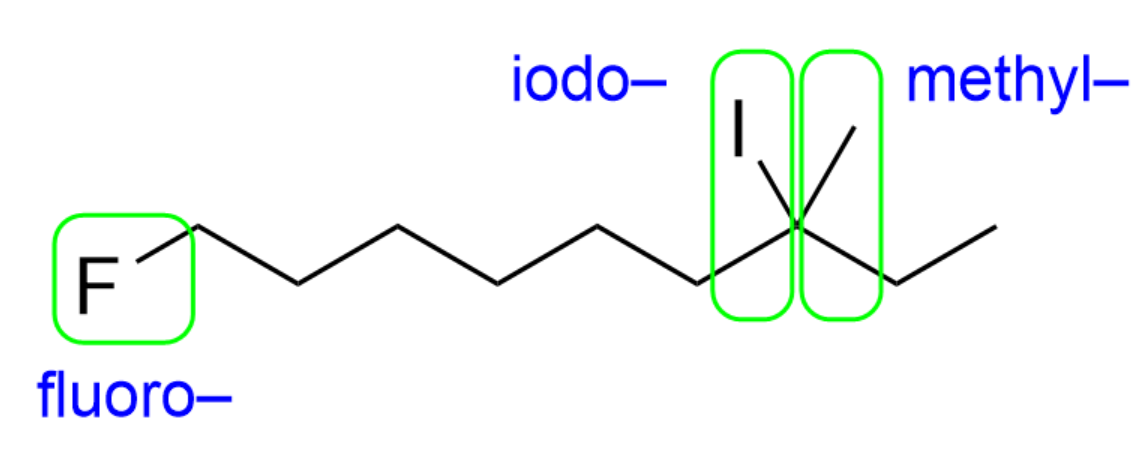
The next step you should take is to identify any branches/substituents. This organic molecule contains a “fluoro-“, “iodo-”, and “methyl-“ substituent.
You should also count how many carbon atoms are in the longest chain, and decide which carbon atom is carbon 1. In this case, the longest carbon chain contains nine carbon atoms yielding the prefix “non-“, while carbon 1 is adjacent to the fluoro substituent. This is because all substituents contain the same priority, and it is the pathway where you encounter a substituent the earliest.
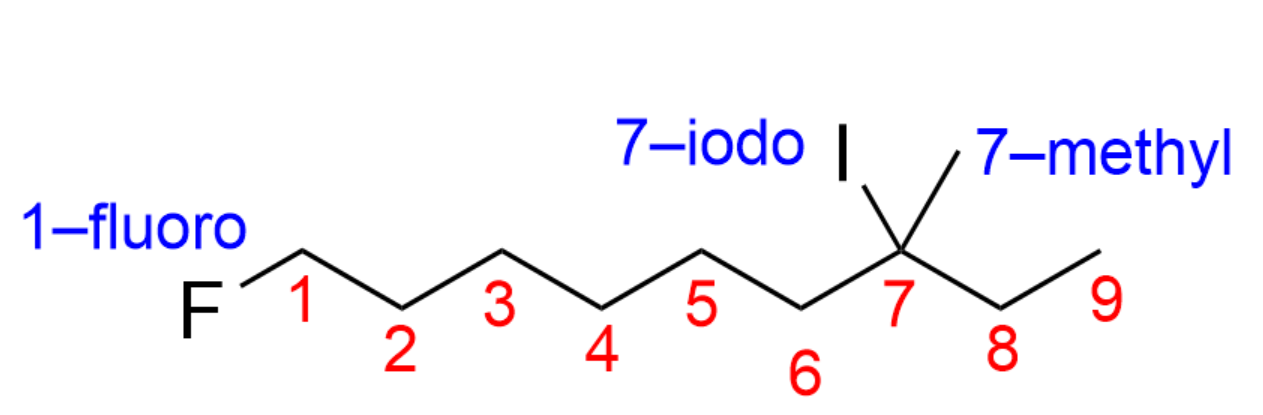
We therefore have “1-fluoro”, “7-iodo” and “7-methyl” as substituent names in that order (alphabetical).
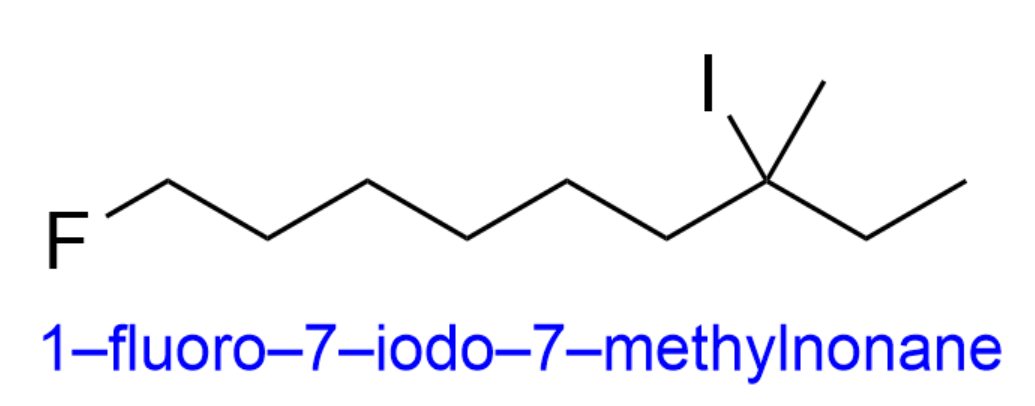
By putting all of this information together, we get the final name, 1-fluoro-7-iodo-7-methylnonane. Therefore, the correct answer is Option C.
3. Which of the following molecule is 2,2-diethyl-3-chlorocyclohexanone?
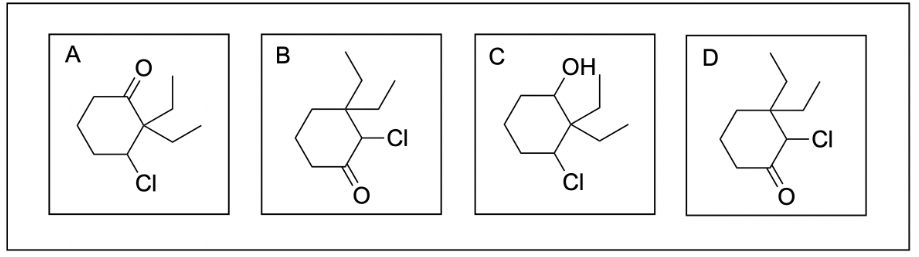
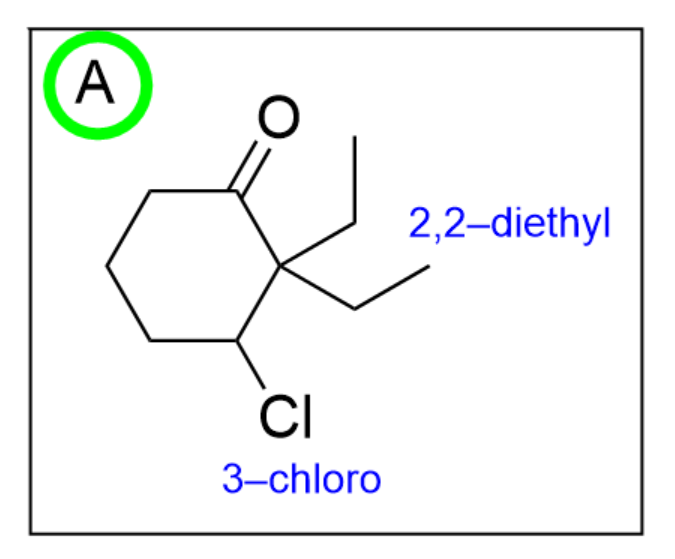
Option A is correct. The first thing you should do is ensure all of the functional groups are present. Based on the final suffix, there should be a ketone present in the molecule, attached to a 6-membered ring (cyclohex-), which we see in this option. Next, you should check if the positions of the substituents are correct. Since the ketone is not numbered, you can assume that the ketone is at position 1. Therefore, the 2 ethyl groups should be 2 carbon atoms away from the ketone while the chlorine atom should be 3 carbon atoms away from the ketone. We see all of these conventions on Option A.
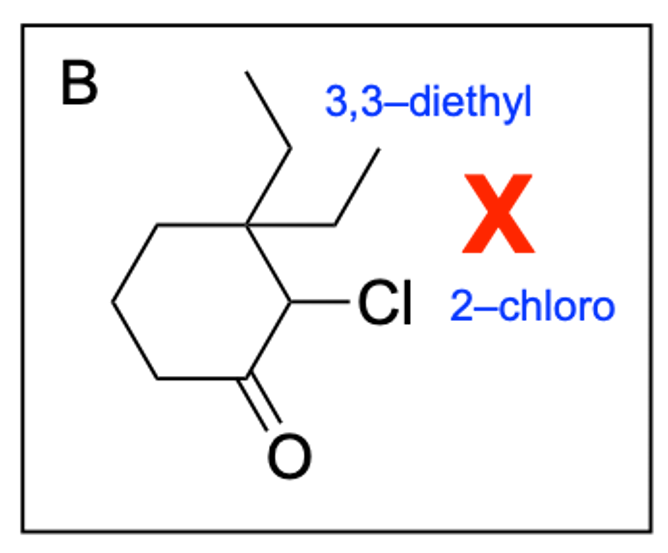
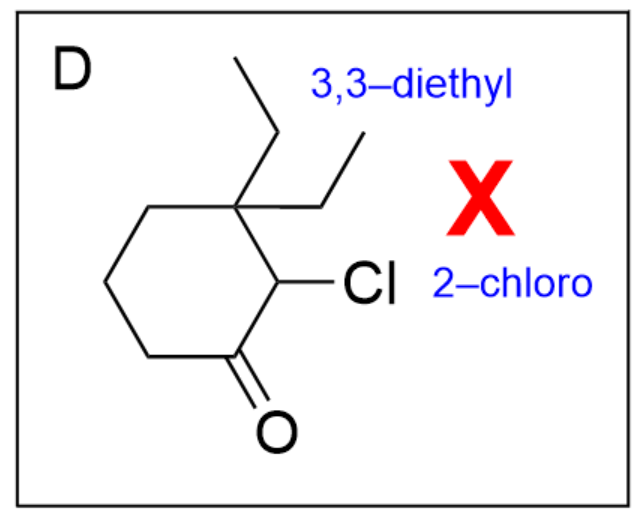
Option B is incorrect. While there is a 6-membered ring with a ketone at position 1 present, the positions of the chloro and diethyl groups are switched (the chloro group should be in position 3 while the diethyl groups should be at position 2).
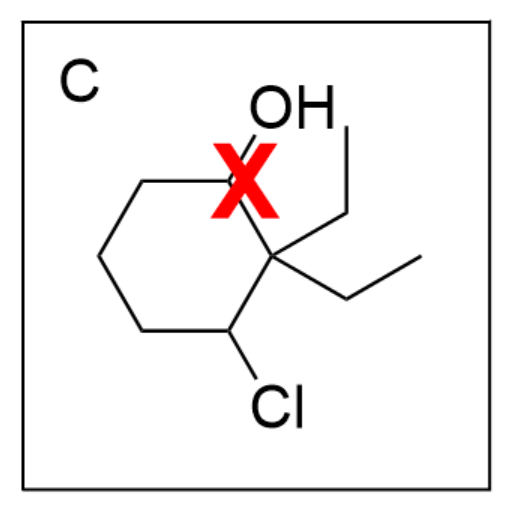
Option C is incorrect. While there is a 6-membered ring present, there is an alcohol instead of a ketone. For this option to be correct, the final suffix should be “-ol”.
Option D is incorrect. While there is a 6-membered ring with a ketone at position 1 present, the positions of the chloro and diethyl groups are switched (the chloro group should be in position 3 while the diethyl groups should be at position 2).
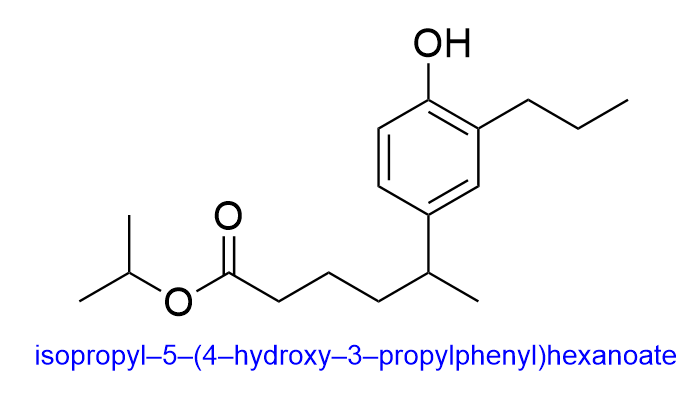
4. What is the name of the following molecule?
A. isopropyl 5-(1-hydroxy-2-propylphenyl)hexanoate
B. propyl 5-(1-hydroxy-2-propylphenyl)hexanoate
C. propyl 5-(4-hydroxy-3-propylphenyl)hexanoate
D. isopropyl 5-(4-hydroxy-3-propylphenyl)hexanoate
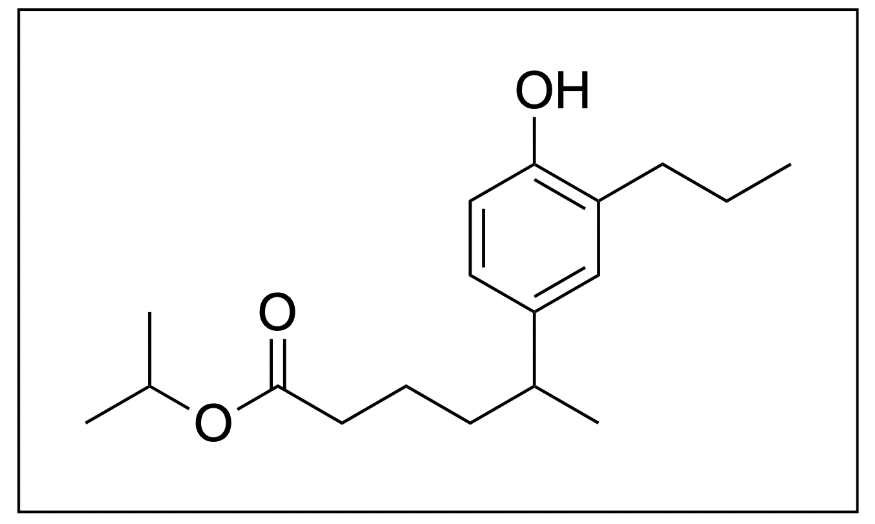
The correct answer to this question is Option D. When naming organic molecules, the first thing you should do is identify the functional groups present to determine priority.
In this molecule, there is an ester, phenyl and alcohol group present. The ester takes priority, so the final suffix should be “-oate”.
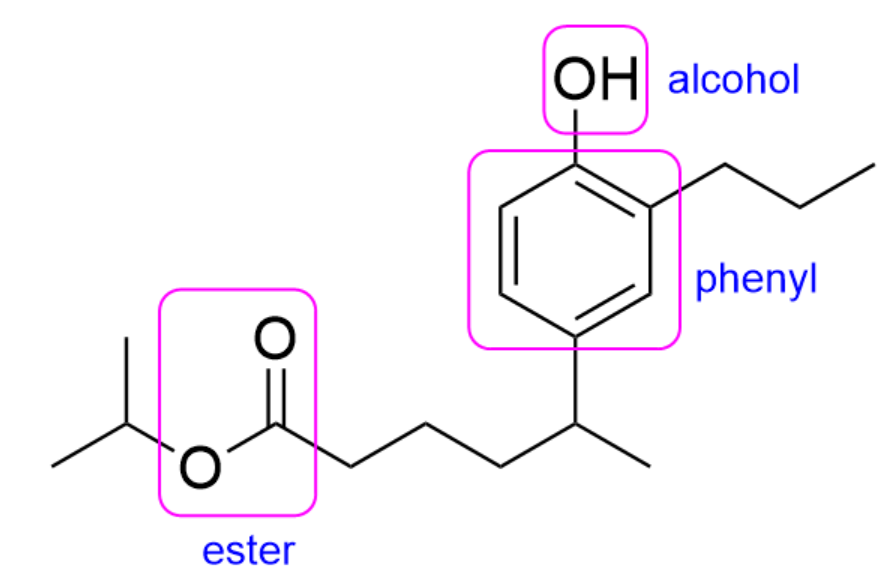 Additionally, when naming esters, the group branching off of the oxygen will be treated as substituents while the chain closest to the carbonyl will be part of the parent chain. Therefore, the substituent name is “isopropyl”, while the 6-carbon long parent chain would be “hexanoate”.
Additionally, when naming esters, the group branching off of the oxygen will be treated as substituents while the chain closest to the carbonyl will be part of the parent chain. Therefore, the substituent name is “isopropyl”, while the 6-carbon long parent chain would be “hexanoate”.
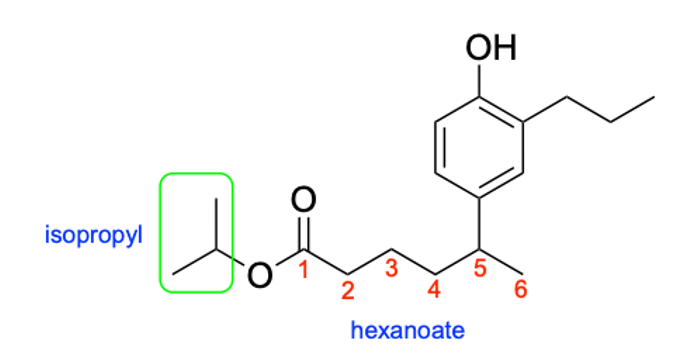
Unfortunately, this molecule also contains branches in the parent chain portion at position 5. We will therefore have to include the name of these substituents in brackets to show that these substituents belong to the carbonyl portion and not the oxygen portion of the ester, and the position in the parent chain where this branch is located.
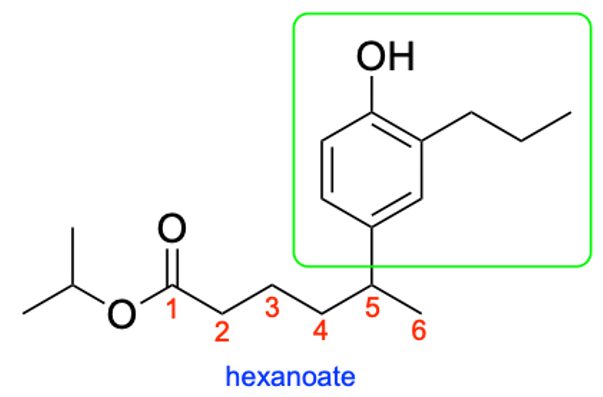
The branch contains a phenyl, alcohol and propyl (3-carbon) group. The phenyl group will take priority and will be named last, while the hydroxy and propyl groups will be alphabetically ordered. The hydroxy group is 4 carbon atoms away from the parent chain while the propyl group is 3 carbon atoms away from the parent chain, yielding the name (4-hydroxy-3-propylphenyl). Additionally, since the parent chain contains branches at position 5, the name of the branch would be 5-(4-hydroxy-3-propylphenyl).
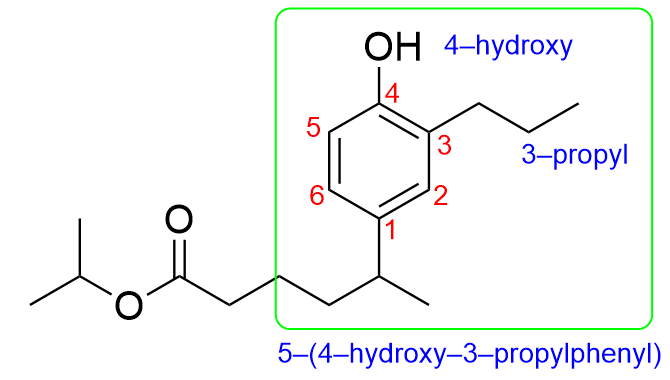
Putting all of this information together, we get the name, isopropyl 5-(4-hydroxy-3-propylphenyl)hexanoate. Therefore, Option D is the correct answer.