5.1 – Solutions for Chapter 2 – Organic Structure and Bonding
Chapter 2.3 – Functional Groups
1. Which two functional groups are not present in the following molecule?
A. carboxylic acid anhydride, alkene
B. amide, phenol
C. amine, ether
D. acid halide, ester
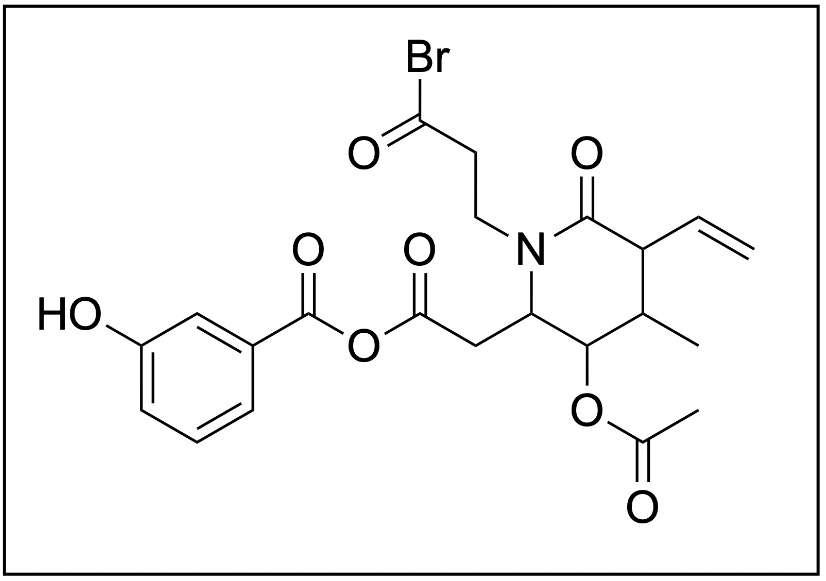
The correct answer to this question is Option C. The first step to this question is to identify the functional groups on the molecule. Below shows an image with each functional group circled and named.
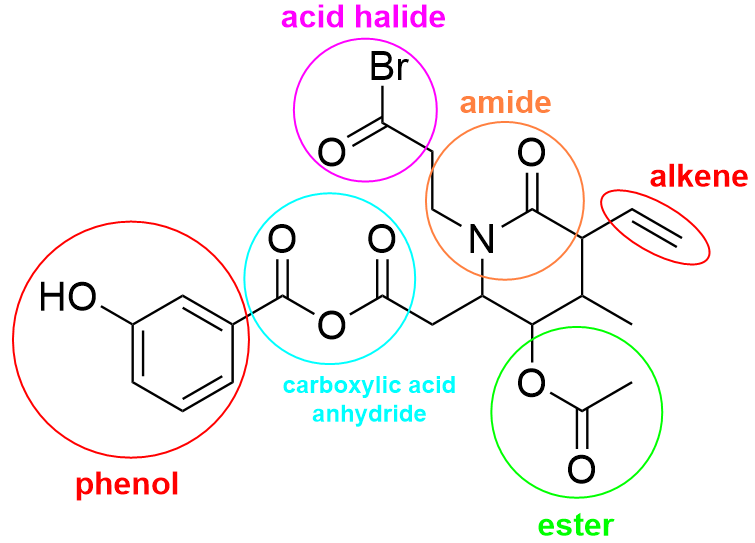
Now that we know what functional groups are present on the compound, we can start going through and eliminating certain options. For example, option A mentions the presence of a carboxylic acid anhydride and an alkene. As both these groups can be found on the molecule (in cyan and red respectively), this option is incorrect. Similarly, option B mentions the presence of an amide and phenol, which are both identified to be on the molecule in orange and red. Option C is also incorrect, as an acid halide and ester are both present on the molecule circled in magenta and green respectively.
Option C mentions the presence of an amine and an ether. Differentiating amines and amides can be difficult, as they both contain a nitrogen group. Remember that amides have a neighboring C=O bond, whereas amines will have nitrogen bonded only to carbon groups (see below). As the nitrogen in this molecule is beside a C=O group, this is considered an amide. Thus, an amine is not present on this molecule.
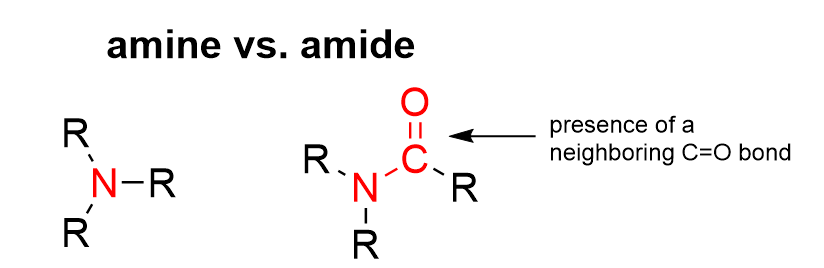
For the ether, recall that it is a functional group that contains an oxygen bonded to two carbon (R) groups on each side, as seen below (R–O–R). This R–O–R arrangement is also observed in an ester functional group and a carboxylic acid anhydride group, which are both found in the compound. However, these are not considered ethers as the oxygen contains either one C=O bond (ester) or two neighboring C=O bonds (carboxylic acid anhydride). This classifies the functional group as something entirely different from an ether. The difference is illustrated below.

Therefore, as both an amide and ether are not on this molecule, Option C is the correct answer.
2. How many primary, secondary, tertiary, and quaternary carbons are there in the following molecule respectively?
A. 12, 9, 6, 6
B. 6, 9, 12, 6
C. 6, 6, 12, 6
D. 12, 6, 6, 6
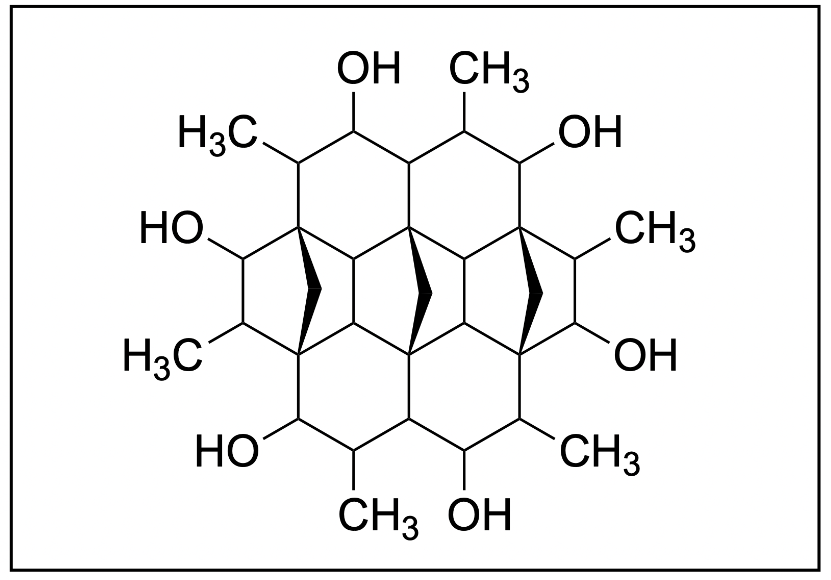
The correct answer to this question is Option B. The best way to solve this question is to count the number of primary, secondary, tertiary and quaternary carbons while keeping track to ensure you don’t mess up and recount carbons. Below details a method of counting carbons to determine the correct option.
You can realistically start counting anywhere on the molecule, but we will start with the outer carbons. Specifically, we will start with the carbons highlighted in green, which are sticking out from the ring shape. The carbon group (–CH3) is only bonded to one other carbon (as shown in the green bond), thus, it is a primary carbon. Going around the entire ring, there are 6 CH3 groups identical to this one, thus we determine that there are 6 primary carbons so far.
Moving one atom inward, highlighted in a cyan circle, there is another carbon. It is bonded to three carbons, as seen by the cyan bonds, and thus, it is a tertiary carbon. Looking at the full molecule, we can see a repeating pattern in the arrangement of carbon atoms. There are 6 carbons in total with this structure, which gives us a total of 6 tertiary carbons.
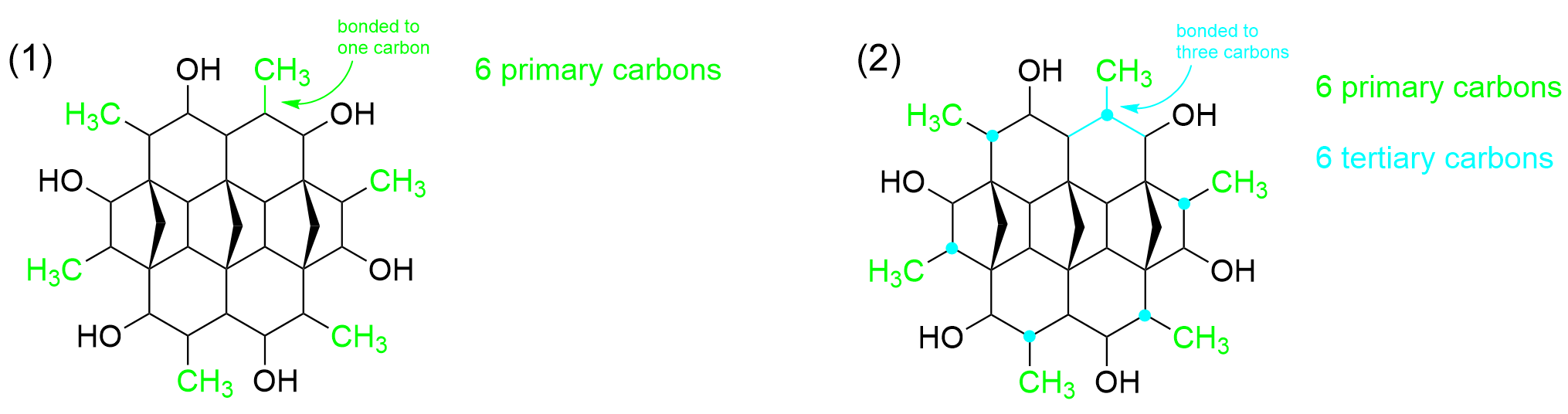
We can then move one atom to the side, highlighted in a dark blue dot, which is bonded to an -OH group. Remember that the classification is based on the number of carbon groups the carbon is bonded to and no other substituents. This carbon group is only bonded to two carbons, as seen by the dark blue bonds, making it a secondary carbon. When we count around the ring, we can see that there are 6 identical carbons with this arrangement, giving us 6 secondary carbons.
Moving to another carbon, as seen by the magenta circle, we can determine that it is a tertiary carbon, as it is bonded to three carbon groups. Looking around, we can find a total of 6 more carbons that are tertiary carbons, bringing up the total number of 12.
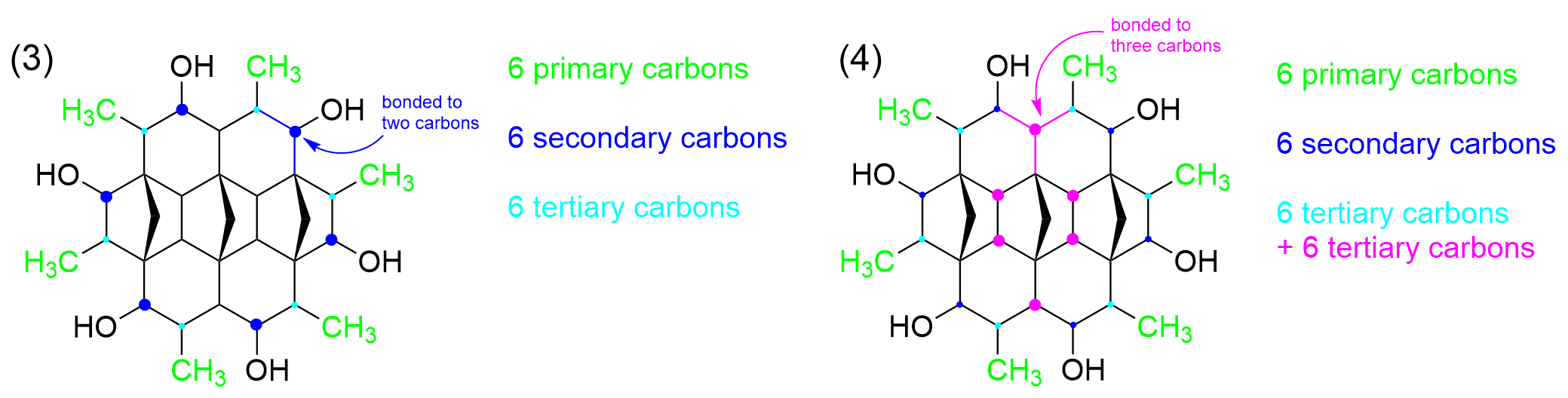
There are also a number of carbons that are bonded to four different carbon groups, which is found in the middle of molecule (red) These give us a total of 6 quaternary carbons.
The only carbons remaining then are in the very middle of the molecule, highlighted in orange. With only two bonds to carbon, these add another 3 secondary carbons to the list, giving us 9 in total.
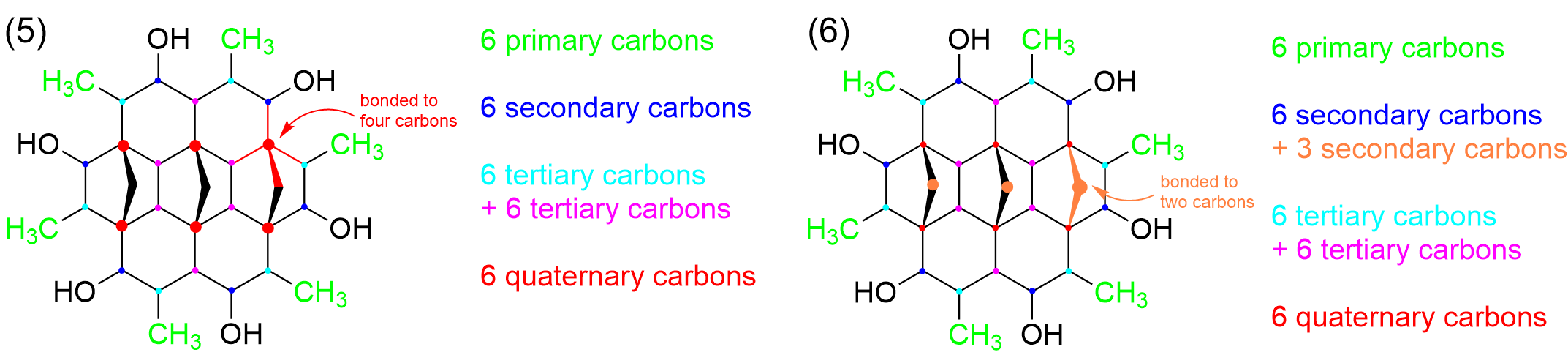
In conclusion, there are 6 primary carbons, 9 secondary carbons, 12 tertiary carbons, and 6 quaternary carbons. This matches up with Option B, the correct answer to this question.

