5.1 – Solutions for Chapter 2 – Organic Structure and Bonding
Chapter 2.2 – Valence Bond Theory
1. The following molecule is called “NanoPutian”, which was synthesized by Professor James Tour in 2003. How many sp3, sp2 and sp hybridized carbons are there in the molecule respectively?
A. 17 ,11, 10
B. 17, 12, 10
C. 16, 12, 9
D. 16, 11, 9
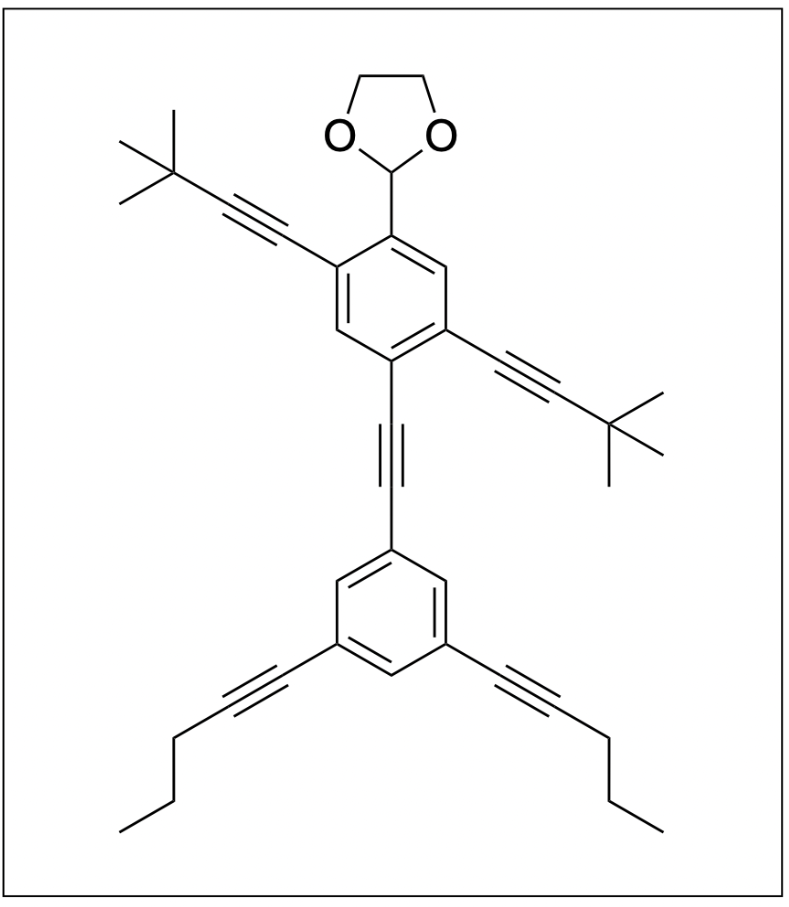
The correct answer to this question is Option B. To determine the hybridization of the various carbon atoms, we can look at how many other atoms the carbon atom is bound to, and we can also use VSEPR theory to help us.
sp3 hybridization involves the mixing of four atomic orbitals, so carbon atoms that are bonded to four other atoms will be sp3 hybridized. Additionally, atoms that have a tetrahedral geometry will also be sp3 hybridized due to the 90.5° bond angles that minimize repulsion.
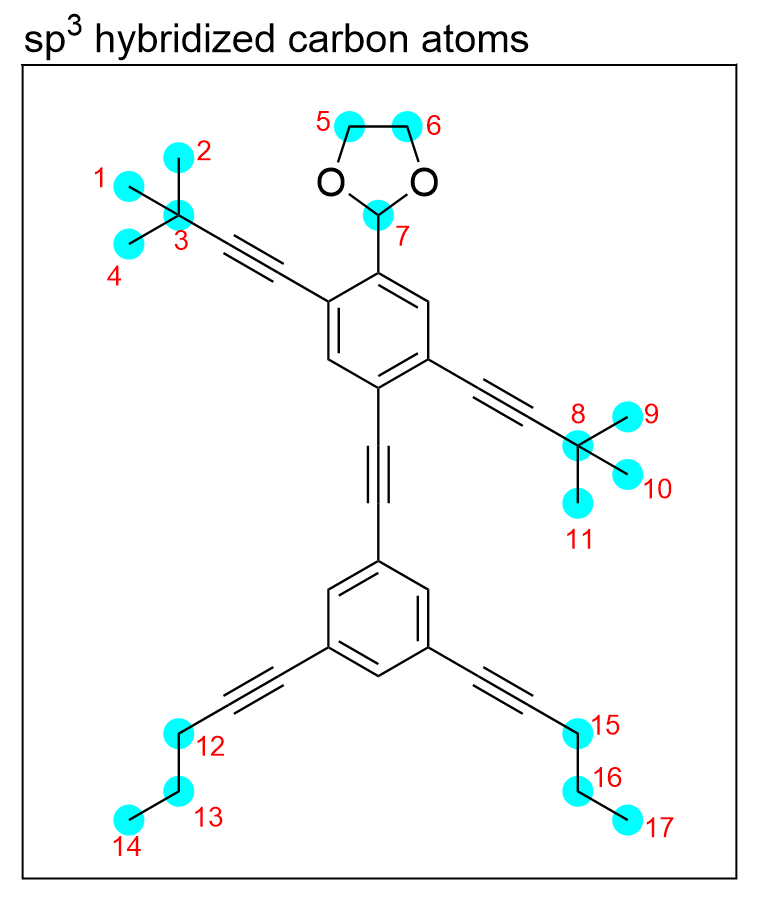
There are 17 carbon atoms (highlighted in blue), that are bonded to 4 other atoms and have tetrahedral geometry, therefore there are 17 sp3 hybridized carbon atoms.
sp2 hybridization involves the mixing of three atomic orbitals, so carbon atoms that are bonded to three other atoms will be sp2 hybridized. Additionally, atoms that have a trigonal planar geometry will also be sp2 hybridized due to the 120° bond angles that minimize repulsion.
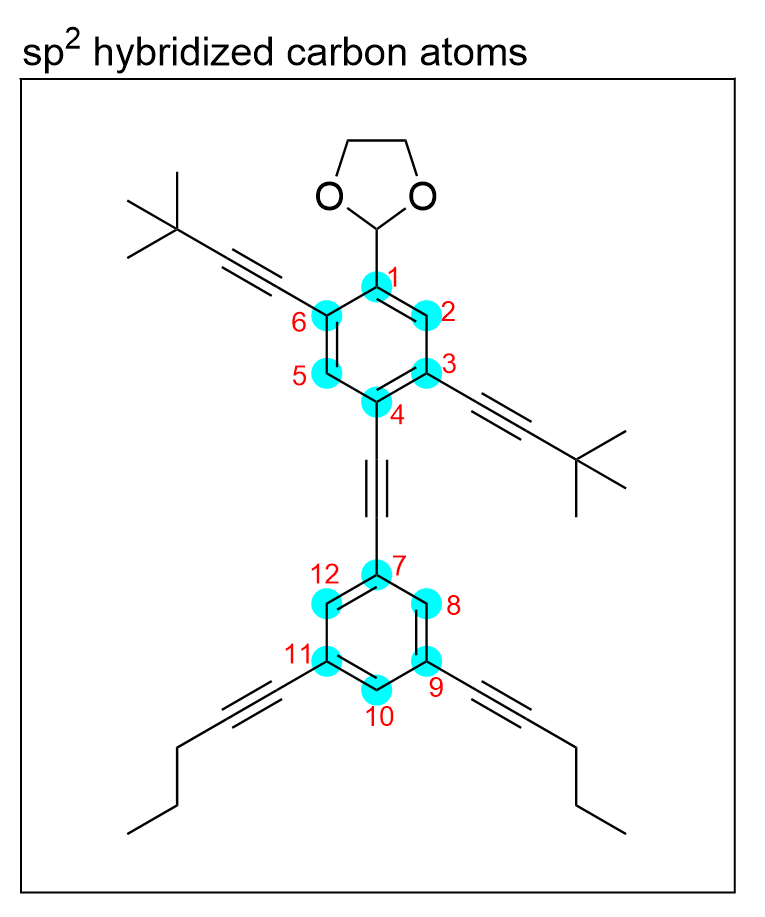
There are 12 carbon atoms (highlighted in blue), that are bonded to 3 other atoms and have trigonal planar geometry, therefore there are 12 sp2 hybridized carbon atoms.
sp hybridization involves the mixing of two atomic orbitals, so carbon atoms that are bonded to two other atoms will be sp hybridized. Additionally, atoms that have a linear geometry will also be sp hybridized due to the 180° bond angles that minimize repulsion.
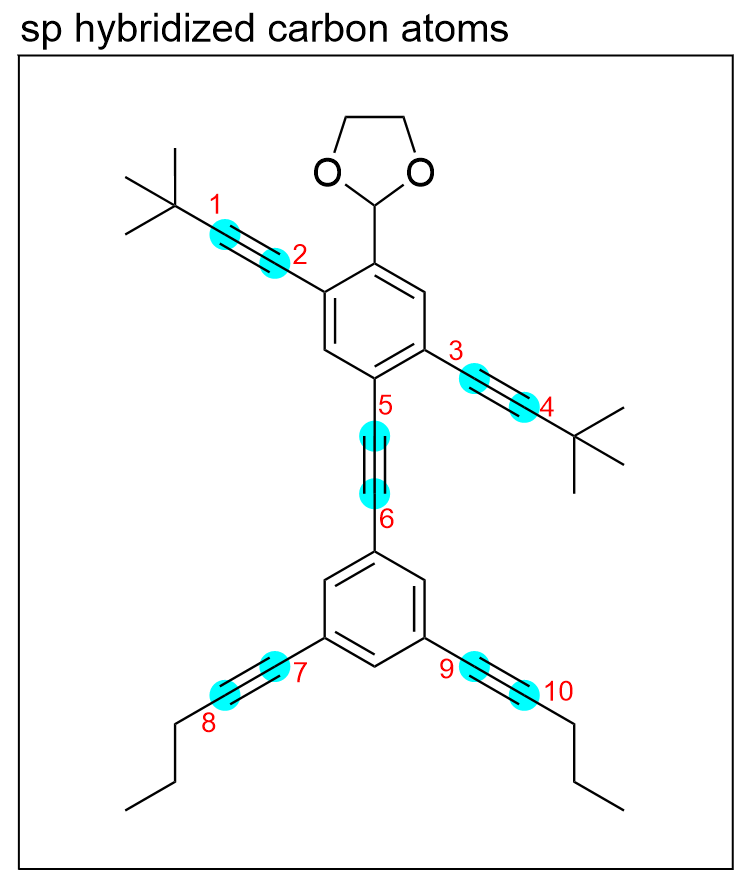
There are 10 carbon atoms (highlighted in blue), that are bonded to 2 other atoms and have linear geometry, therefore there are 10 sp hybridized carbon atoms.
Therefore, the correct answer is Option B.
2. The following molecule is called “NanoPutian”, which was synthesized by Professor James Tour in 2003. How many carbons have a bond angle of 120º?
A. 22
B. 10
C. 17
D. 12

The correct answer to this question is Option D. Recall that only are sp2-hybridized carbons have a bond angle of 120º. In order to minimize repulsion between the three bonds to carbon, the bonds are separated by 120° to maximize the distance between each bond. Sp3 and sp-hybridized atoms also rearrange to minimize repulsion; however, with 4 and 2 bonds on the atom respectively, their bonds angles are at 109.5° and 180°. Thus, to answer this question, we must identify all sp2-hybridized carbons. There are 12 carbon atoms (highlighted in blue), that are bonded to 3 other atoms and have trigonal planar geometry. Therefore there are 12 sp2 hybridized carbon atoms.


