4.4 – Combinatorial Chemistry
Introduction to Combinatorial Chemistry
Once an assay has been developed for testing HVA-like compounds for dopamine inhibition, the next step is to synthesize a library of test compounds to screen. As HVA is the lead compound (Figure 4.4.a), the goal is to synthesize thousands of new compounds that share the core aromatic structure but differ in other aspects. This is done through an approach known as combinatorial chemistry. Combinatorial chemistry is the mixing and matching of substituents at different sites on the molecule to create libraries of unique compounds.
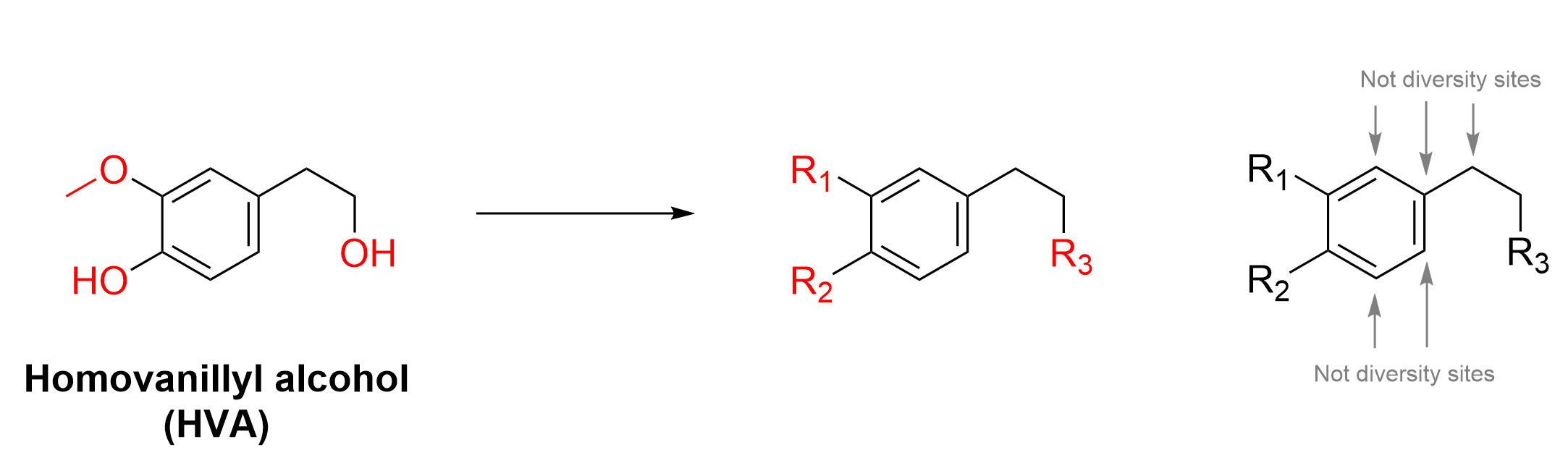
This can be applied to HVA through the manipulation of the diversity sites on the compound. Diversity sites are parts of a molecule which can be substituted with different atoms or functional groups. The molecule HVA below has several diversity sites, three of which are labelled as R1, R2 and R3 (Figure 4.4.b).
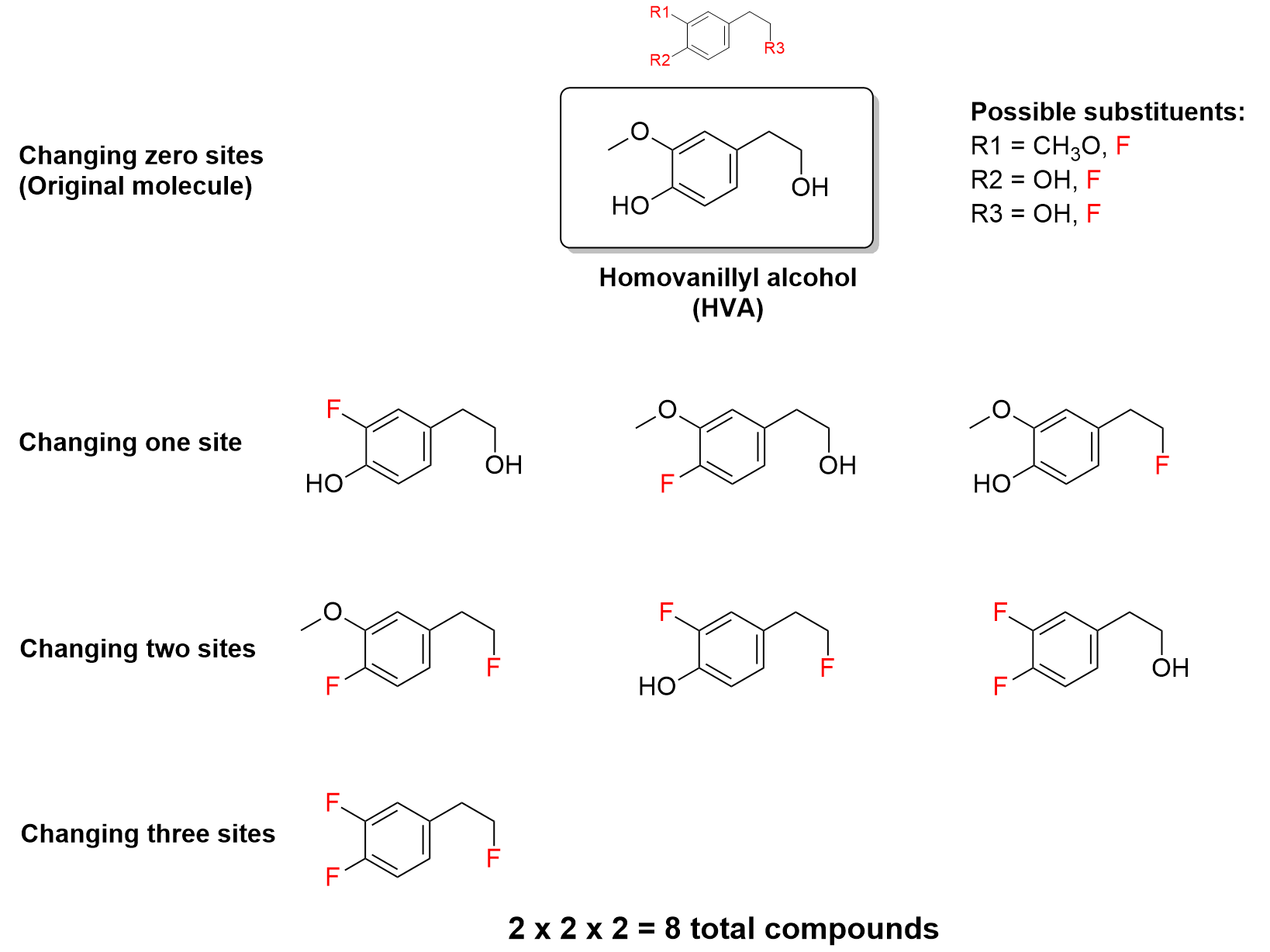
With these three diversity sites, various functional groups or substituents can be added to one or more of them to make different compounds. For example, one substituent, fluorine (F), can be substituted at one or more of the sites to make 8 unique compounds, including HVA.
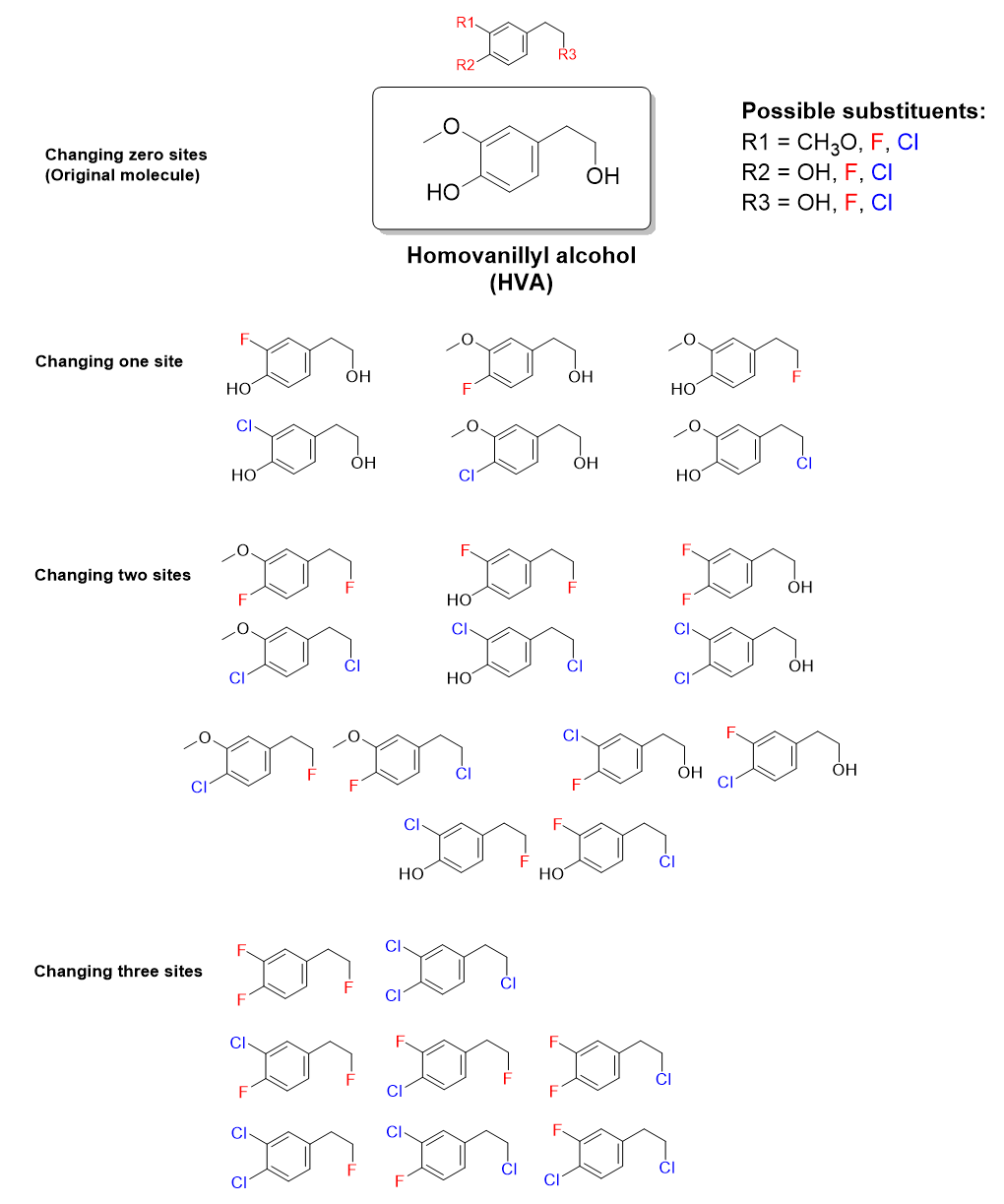
To further expand this idea, we can introduce a second functional group to substitute at each site of diversity, such as chlorine (Cl). Now there are even more possibilities for mixing-and-matching (Figure 4.4.d).
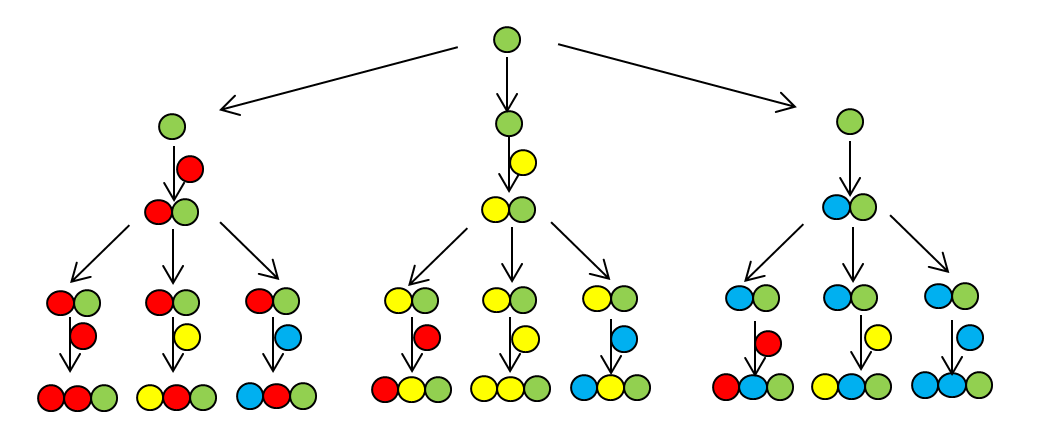
The complete set of compounds that can be produced is called a combinatorial library. The library size refers to the total number of compounds in the library. It is a function of both the number of substituents and the number of diversity sites, expressed in the equation:

The equation for the size of the combinatorial library derives from combinatorics. For example, in Figure 4.4.c, there are two different substituents at three diversity sites. To create any molecule in the library, you have two choices for the first diversity site, two choices for the second diversity site, and two choices for the third diversity site. Therefore, there are 2x2x2 = 8 different possibilities.
For a molecule to become an approved drug, the typical success rate is approximately one in 5 000 to 10 000. Therefore, chemists must create libraries of at least 5000 compounds to screen. This is where combinatorial chemistry plays an important role. By increasing the number of substituents at each diversity site, thousands of novel drug candidates can be produced. All these compounds are synthesized by automated robotic processes, which simplifies and speeds up the synthetic process.
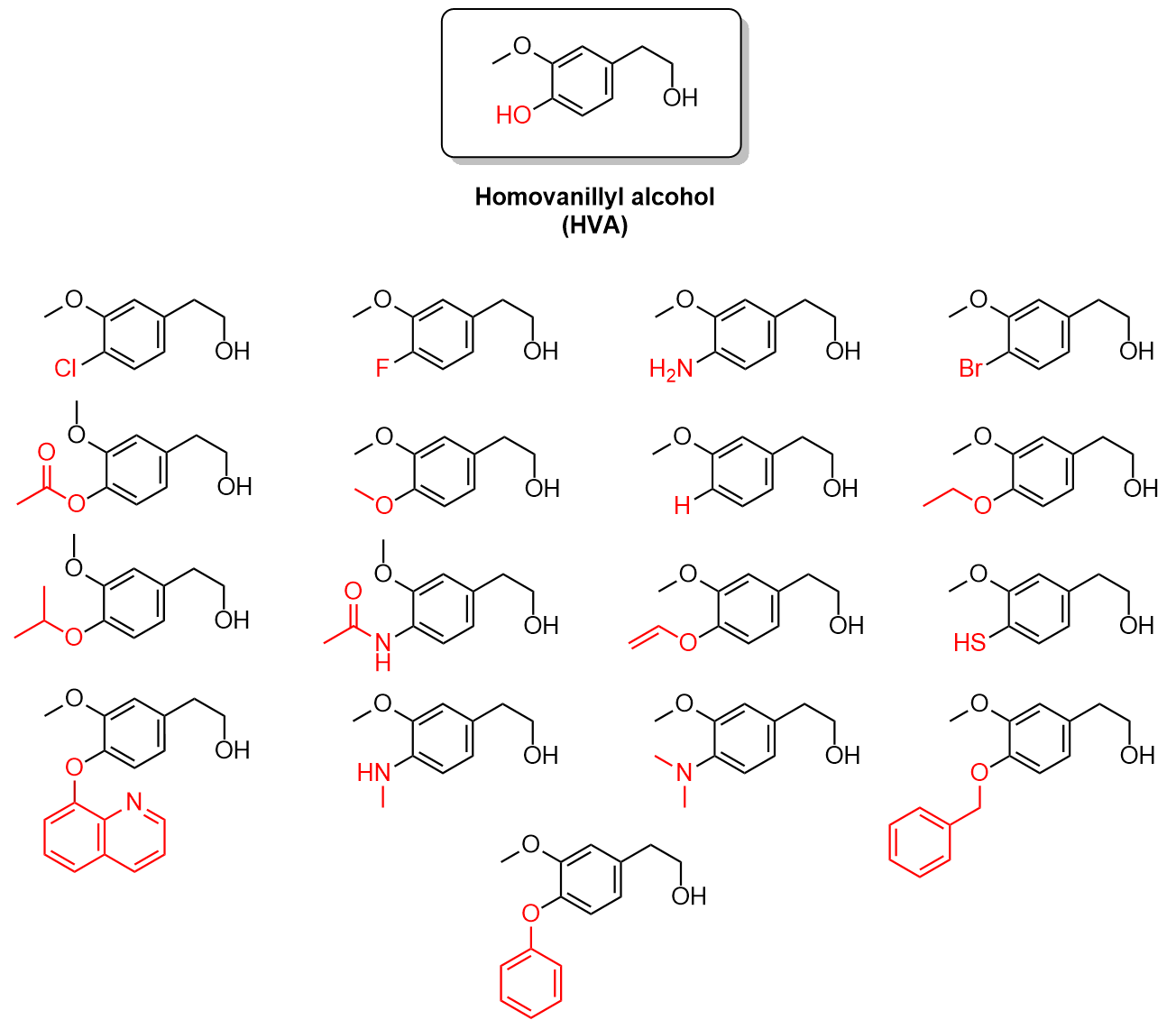
For example, with 18 different substituents at each site, a total of 5832 unique compounds can be synthesized (183 = 5832), all of which can be screened for dopamine inhibition in the hopes of developing a potential therapeutic. Figure 4.4.e below shows a sample of 18 substituents that could potentially be used for this process.
(The full solution this solution can be found in Chapter 5.3)
Identifying Diversity Sites on a Library of Molecules
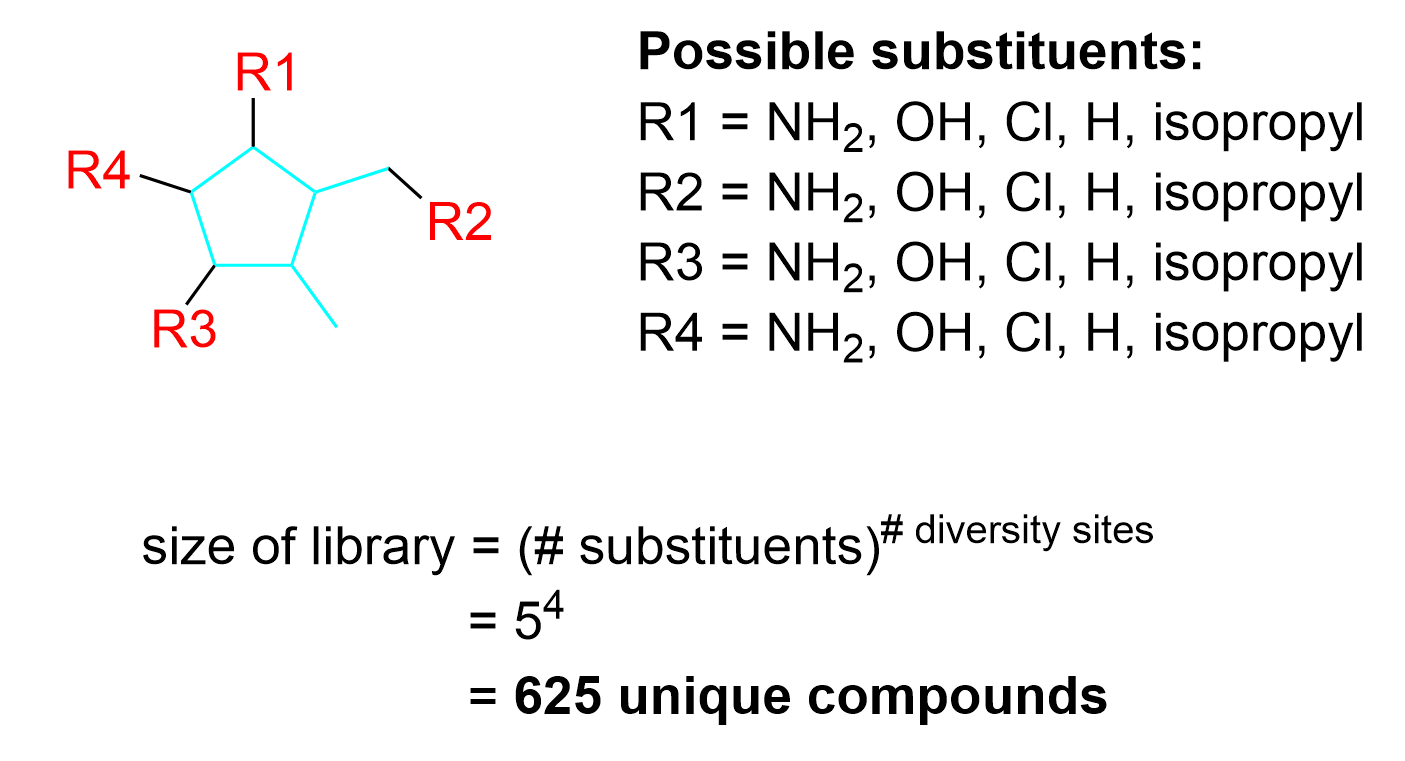
Given several molecules in the combinatorial library, it is possible to infer the number of substituents and diversity sites present, and calculate the maximum size of the combinatorial library. For example, three molecules in a library of drug candidates are shown in Figure 4.4.f.
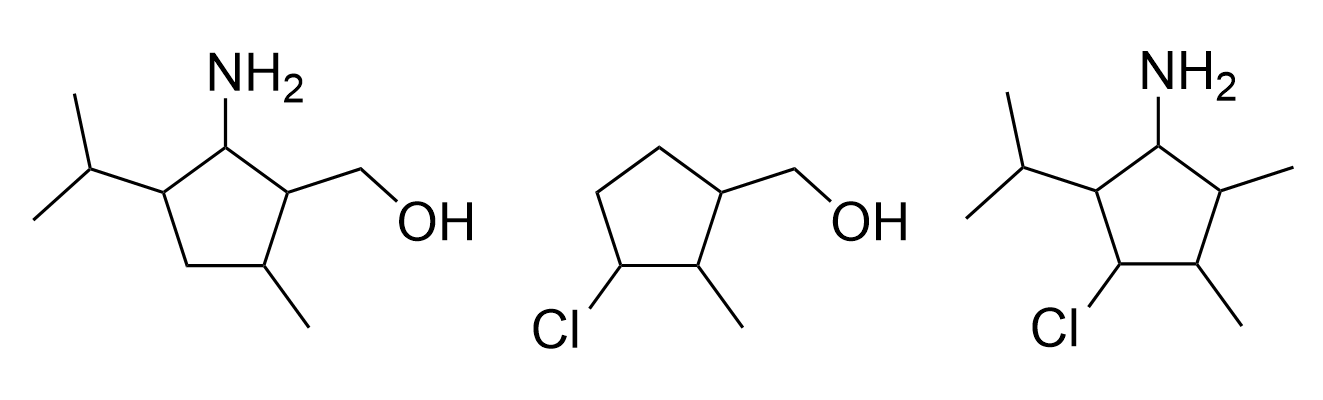
To determine the number of diversity sites, the first step is to determine the core structure that stays constant throughout all the molecules. In this example, the core structure includes carbon atoms within the ring and outside of the ring, shown in blue in Figure 4.4.g.
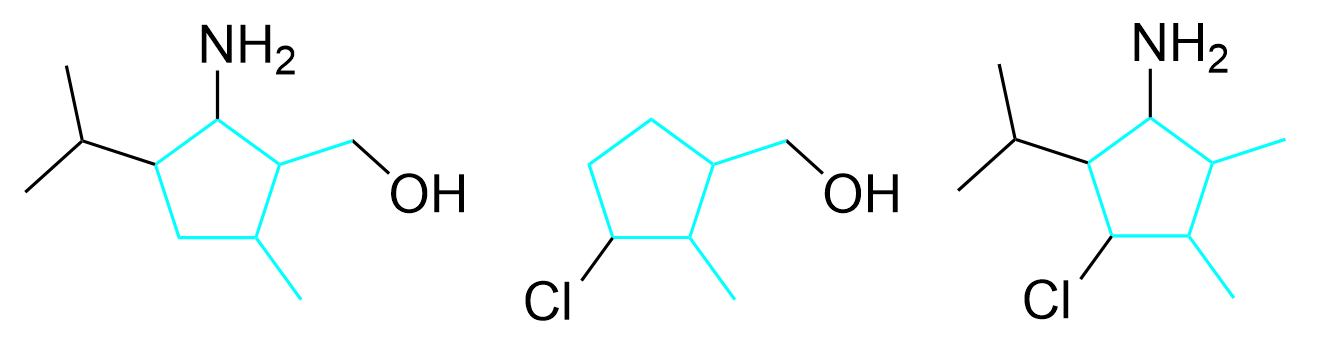
After determining the core structure, the diversity sites can be identified (Figure 4.4.h). These are the locations that have different substituents in the three molecules. For example, diversity site 1 could have the substituent NH2 (in the molecules on the left and right) or the substituent H (in the molecule in the center). Similarly, diversity site 2 could have the substituents OH or H, diversity site 3 could have the substituents Cl or H, and diversity site 4 could have the substituents H or isopropyl. The carbon group on the bottom right highlighted by the gray arrow is commonly mistaken to be a diversity site as it is a substituent outside of the ring; however, as this carbon group shows up in all three molecules, it is not changing and thus, is not a diversity site.
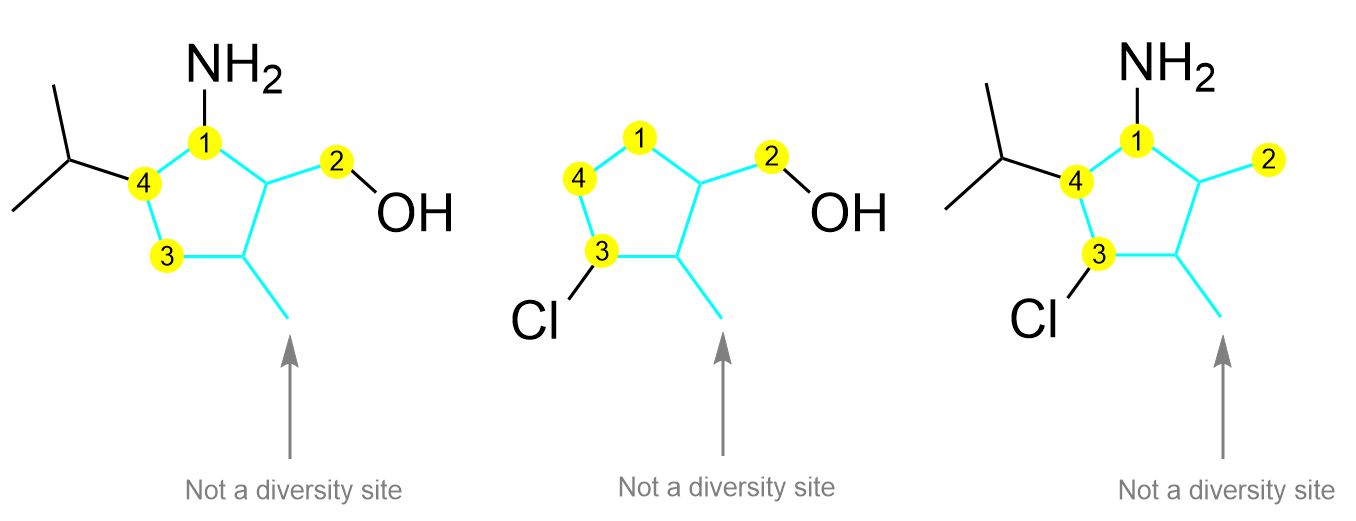
The next step is to identify the different substituents that are used to create this library. In these three compounds, there are a total of five different substituents present on the various diversity sites: NH2, OH, H, Cl, isopropyl. Thus, when constructing a full library of drug candidates, all five of these substituents can be used on each diversity site. Thus, a total of 54 = 625 unique compounds can be synthesized.
The following video includes a worked example from a previous CHEM 1AA3 test or exam that students struggled with. Try solving it on your own before looking at the solution.
(The full solution to this problem can be found in Chapter 5.3)
Key Takeaways
- In the drug delivery process, combinatorial chemistry can be utilized on a lead compound of choice, such as HVA, to create a library of compounds to test.
- Combinatorial chemistry involves “mixing and matching” various substituents onto specific atoms of the lead compound called diversity sites. These sites can have the functional groups modified or substituted to other substituents of choice.
- The resulting library of different but similar compounds is called a combinatorial library.
- The amount of compounds in a library is given by the equation below:
- (# substituents)(# diversity sites)
Key terms in this chapter:
| Key term | Definition |
| Combinatorial chemistry | The process of modifying a lead compound with various functional groups at multiple sites to create a large library of compounds. |
| Diversity sites | An atom on a lead compound which can have its functional groups modified to other functional groups of choice. |
| Combinatorial library | A library, or large collection, of unique compounds, all with the same core structure derived from a lead compound. |
Diversity in Chemistry: Árpád Furka
The field of combinatorial chemistry is a lot broader than discussed in this chapter, with not only diverse small molecules being synthesized, but also larger polymers made of amino acids and nucleotides. Árpád Furka is considered one of the pioneers of combinatorial chemistry, developing a method known as the split-and-mix synthesis. This technique uses solid-phase synthesis, where a growing peptide is bonded to a solid bead, and reactants are added to the resin to react with the reactive growing chain. As the name suggests, different combinations of amino acids can be made, and then these small chains can be divided and react with each other to form larger ones. The cycle can repeat infinitesimally to synthesize millions of different combinations of peptides or DNA for testing. This technique was first developed in 1982 at the Eötvös Loránd University in Budapest, Hungary. As a young boy born into poverty, Furka faced many challenges as he mainly worked in his adolescence to support his family, and was behind his peers in academics as he did not have the opportunity to study. Despite so, after attending school in his early twenties, Furka managed to become a trailblazer in combinatorial chemistry, developing one of the most powerful techniques in the field.
Any feedback or comments on this chapter? You may either email chemoer@mcmaster.ca, access this MS Form, or provide a comment in the feedback box below.
The process of modifying a lead compound with various functional groups at multiple sites to create a large library of compounds.
An atom on a lead compound which can have its functional groups modified to other functional groups of choice.
A library, or large collection, of unique compounds, all with the same core structure derived from a lead compound.

