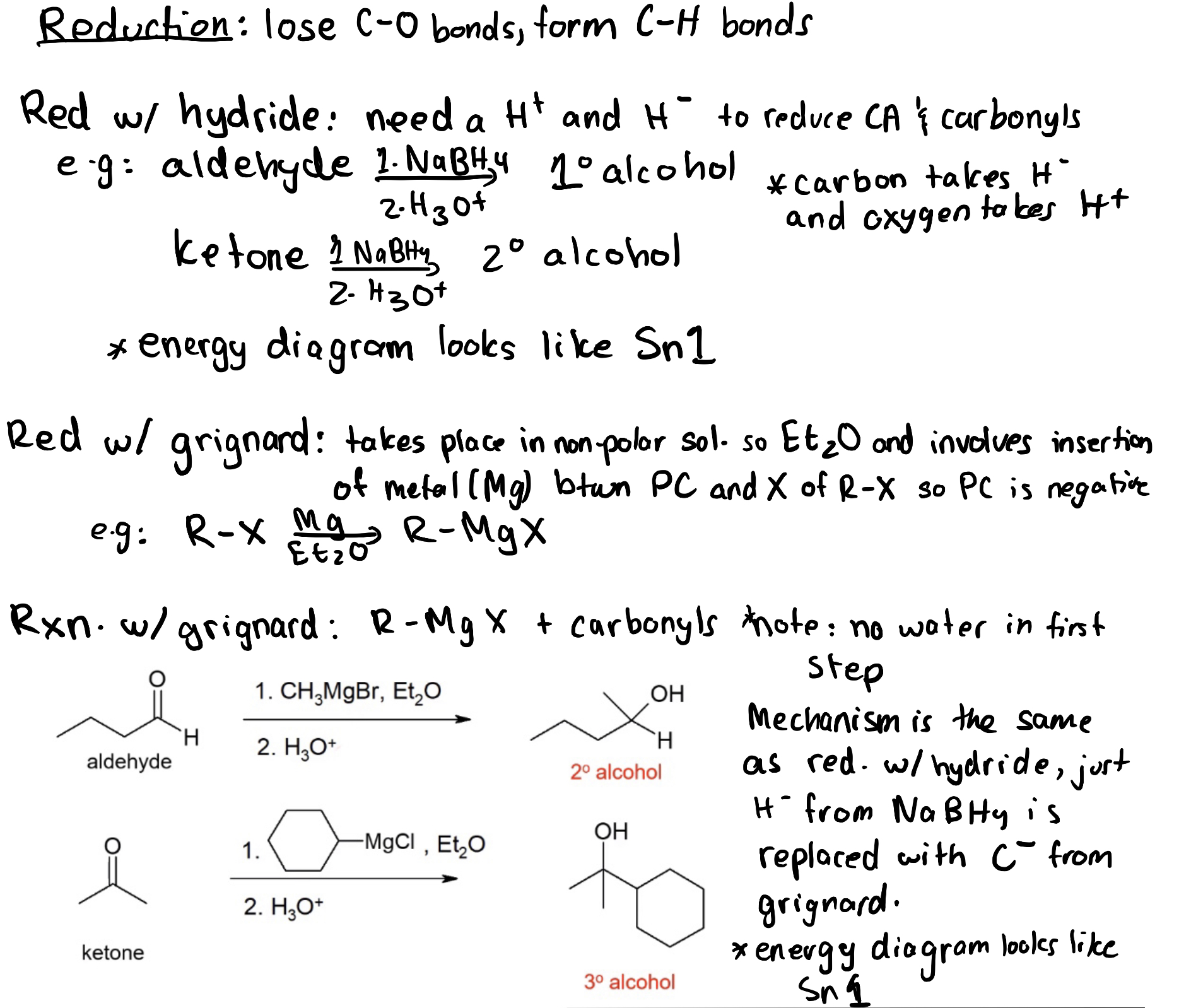
3.4.3 – Comparing Sodium Borohydride Reductions and Grignard Reactions
The last two chapters discussed the reduction of carbonyl groups using sodium borohydride and the addition of a carbon-containing group using a Grignard reagent. Although these reactions may seem different at first glance, they have several similarities.
First, both reactions result in the formation of alcohol.
Second, both reactions result in the reduction of the carbonyl group. The C=O π bond is broken, and two new σ bonds are formed: an O-H σ bond and either a C–H σ bond (for sodium borohydride reduction) or a C–C σ bond (for the Grignard reaction).
Third, both reactions follow a two-step process. The first step involves attack of electrophilic carbon in the carbonyl group by the nucleophilic hydride (for the sodium borohydride reduction) or the nucleophilic Grignard reagent (for the Grignard reaction). The intermediate in both reactions is an anionic alkoxide (Figure 3.4.3.a). The second step (Figure 3.4.3.b) is the same for both reactions, involving an acid-base reaction between the anionic alkoxide (base) and hydronium (acid).
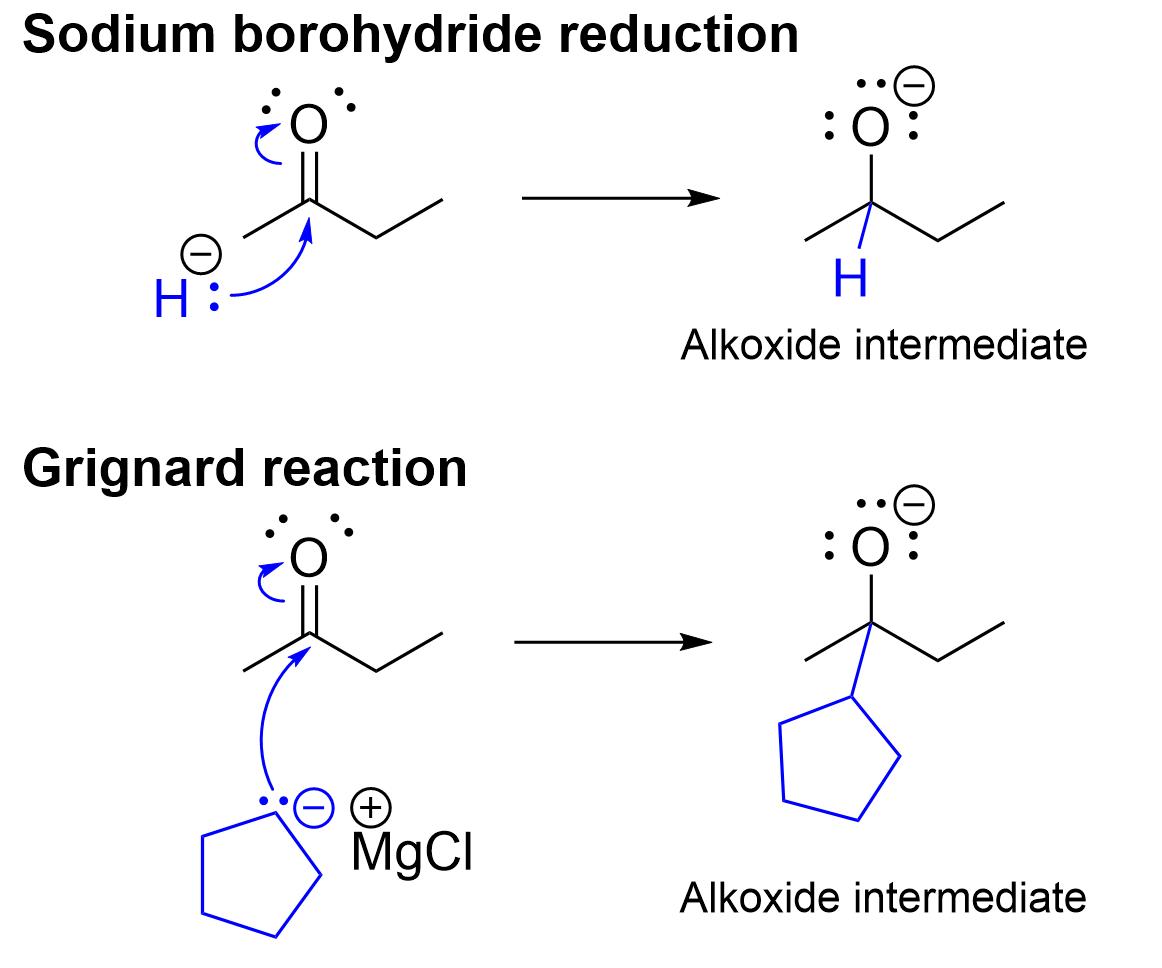
The difference between these reactions is that the Grignard reaction adds a new carbon-containing group whereas sodium borohydride a hydrogen to the carbon centre of the carbonyl group.
Table 3.4.3. A comparison between the sodium borohydride reduction of carbonyls and the Grignard reaction with a carbonyl group.
| NaBH4 Reduction of Carbonyls | Grignard Reaction with Carbonyls | |
| Product | Formaldehyde → Methanol
Aldehyde → Primary alcohol Ketone → Secondary alcohol |
Formaldehyde → Primary alcohol
Aldehyde → Secondary alcohol Ketone → Tertiary alcohol |
| Bonds Broken and Formed | C=O π bond is broken
C–H σ bond is formed O–H σ bond is formed |
C=O π bond is broken
C–C σ bond is formed O–H σ bond is formed |
| # of Mechanistic Steps | 2 steps; first step is slow | 2 steps; first step is slow |
| Intermediate | Anionic alkoxide | Anionic alkoxide |
| First Step | Attack of electrophilic carbon in the carbonyl group by the nucleophilic hydride | Attack of electrophilic carbon in the carbonyl group by the nucleophilic Grignard reagent |
| Second Step | Proton transfer from hydronium (acid) to alkoxide (base) | Proton transfer from hydronium (acid) to alkoxide (base) |
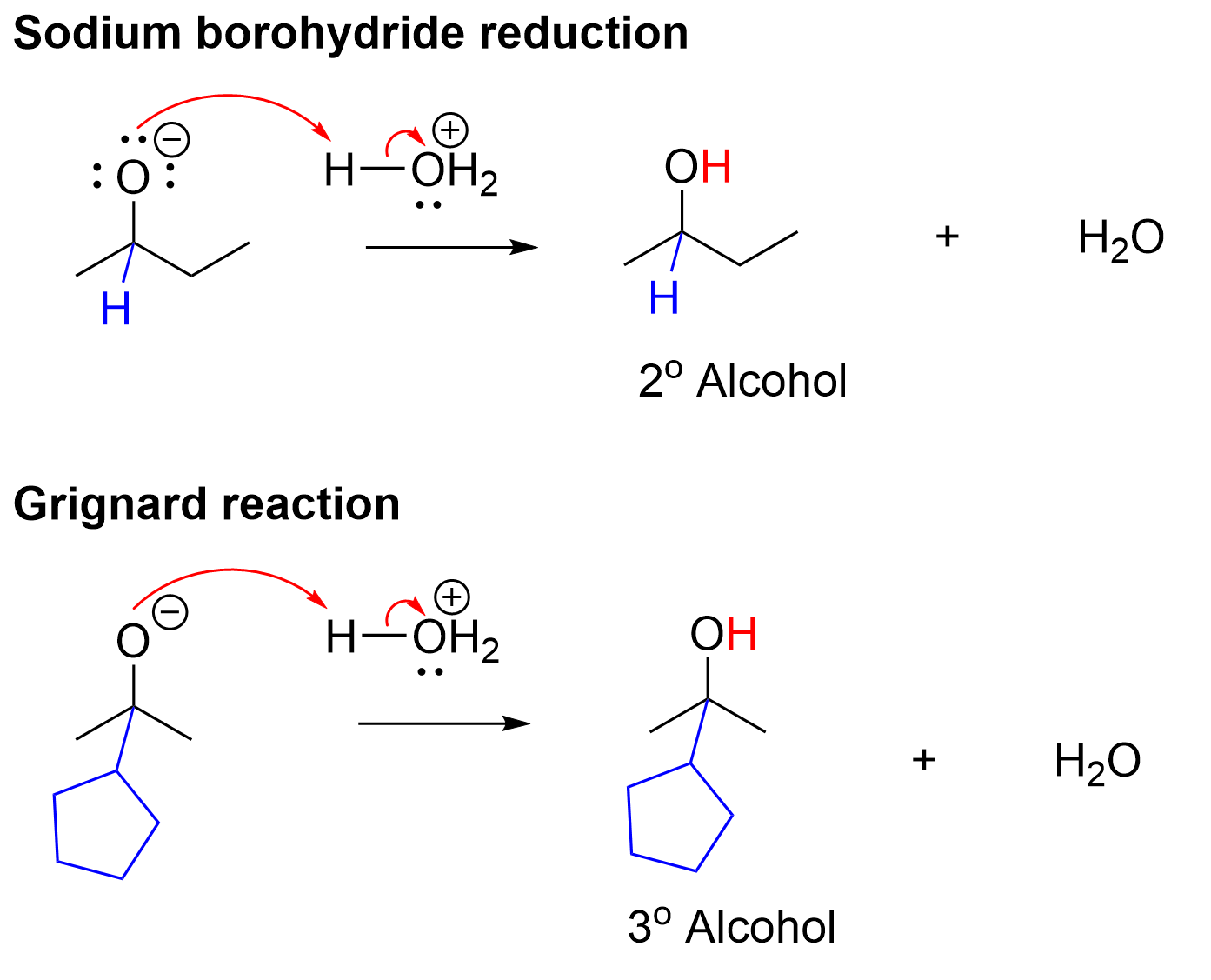
The second step in both reactions are the exact same, in that the alkoxide must be protonated using mild acid. In both, the lone pair on the alkoxide abstracts a hydrogen from the H3O+ acid, leading to our final alcohol product. Note that the reduction results in a secondary alcohol, whereas the Grignard reaction results in a tertiary alcohol.
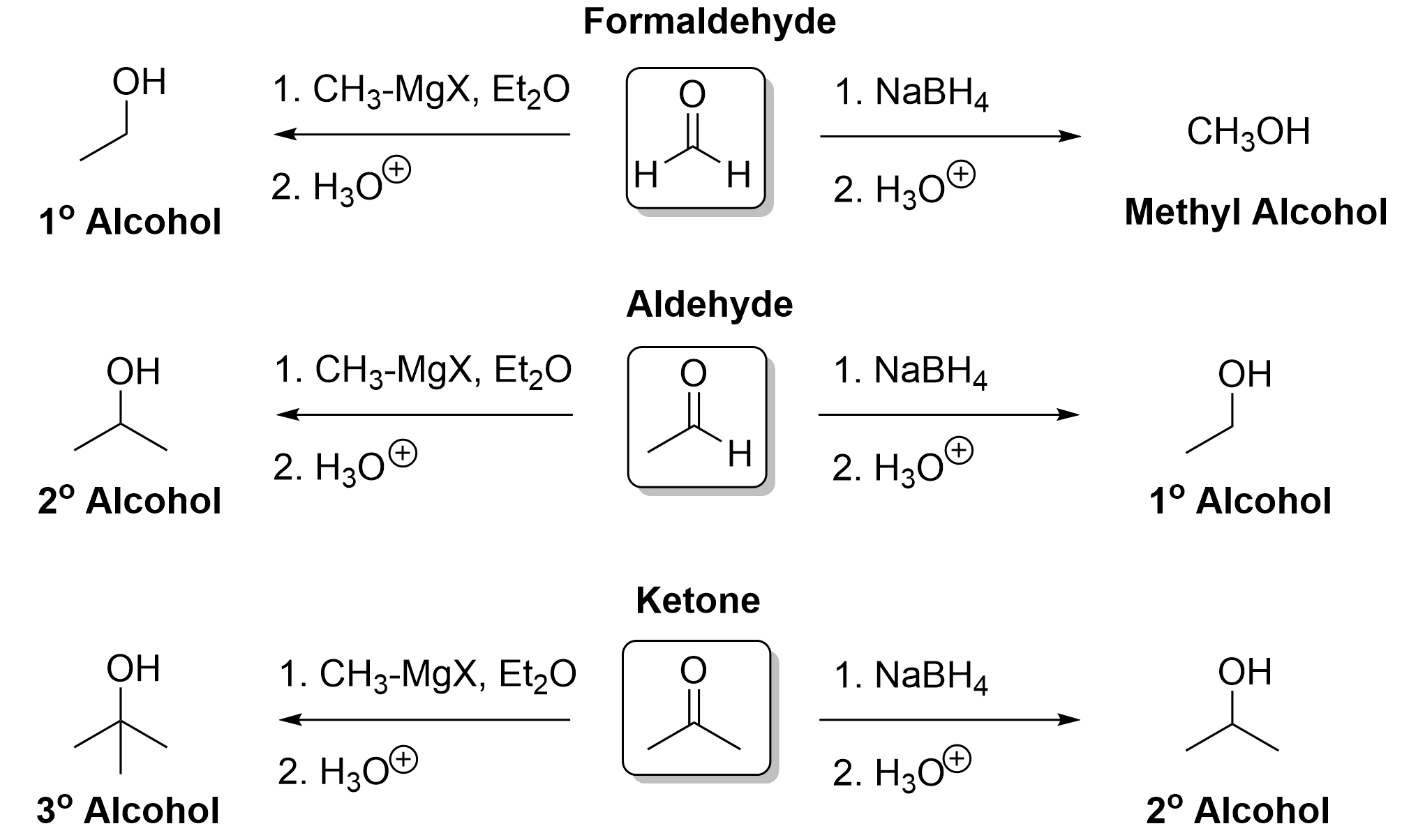
Discerning Between Sodium Borohydride Reduction and Grignard Reaction
To determine whether to use NaBH4 reduction or a Grignard reaction to produce an alcohol from an aldehyde or ketone, you can take two different approaches (Figure 3.4.3.c).
The first method is to determine whether the number of carbon atoms has increased in the products relative to the reactants. If the number of carbon atoms is the same, then this is NaBH4 reduction; if the number of carbons has increased, then this is a Grignard reaction.
The second method is to identify the functional groups, including whether the starting material is an aldehyde or a ketone, and whether the product is a primary, secondary, or tertiary alcohol. If an aldehyde reacts to form a primary alcohol, then this is NaBH4 reduction. If an aldehyde reacts to form a secondary alcohol, then this is a Grignard reaction. If a ketone reacts to form a secondary alcohol, then this is NaBH4 reduction. If a ketone reacts to form a tertiary alcohol, then this is a Grignard reaction.
(The full solution to this problem can be found in Chapter 5.2)
Study Notes – Chapter 3.4
Any feedback or comments on this chapter? You may either email chemoer@mcmaster.ca, access this MS Form, or provide a comment in the feedback box below.

