3.2 – Alkene Addition Reactions
- Predict products and write reaction schemes for the following reactions (no curved arrow mechanisms or energy profile diagrams required):
- Hydrogenation of an alkene or alkyne
- Halogenation of an alkene
- Predict products, write reaction schemes, draw curved arrow mechanisms, and draw energy profile diagrams, including all intermediates and transition states, for the following reactions:
- Hydrohalogenation of an alkene
- Acid-catalyzed hydration of an alkene
- Understand the relationship between the curved arrow mechanism, energy profile diagram, and rate law for the addition reactions to alkenes.
- Explain trends in carbocation stability (primary, secondary, tertiary) and why carbocation stability would favour certain products (i.e., the chemical basis for Markovnikov’s rule).
Alkene Reactivity
Alkenes undergo a wide variety of reactions that share some common features due to the electron dense carbon-carbon double bond. This reactivity makes alkenes an important type of organic compound because they can be used to synthesize a wide variety of other compounds. The most common type of reaction for alkenes is the addition reaction to a C=C double bond. In addition reactions, a small molecule is added across a π bond. As a result, one π bond and one σ bond are broken, and two σ bonds are formed. This also means that the hybridization of the atoms in the molecule changes – for addition reactions of alkenes, the carbon atoms in the C=C double bond of reactants are sp2 hybridized, and they become sp3 hybridized in the products.
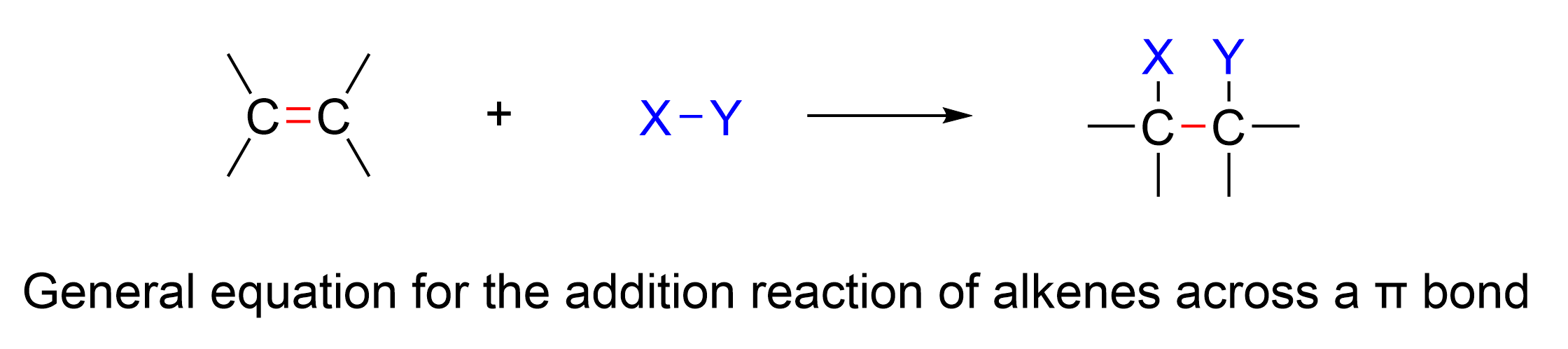
This chapter will focus on the various addition reactions that alkenes undergo, and the chapter is divided based on which molecule is added across the π bond.
Key terms in this chapter:
| Key term | Definition |
| Addition reactions | A small molecule (such as water) is added to a multiple bond (a double or a triple bond). This results in one π–bond and one σ–bond being broken and two new σ–bonds being formed. |
Any feedback or comments on this chapter? You may either email chemoer@mcmaster.ca, access this MS Form, or provide a comment in the feedback box below.
A small molecule (such as water) is added to a multiple bond (a double or a triple bond). This results in one π–bond and one σ–bond being broken and two new σ–bonds being formed.

