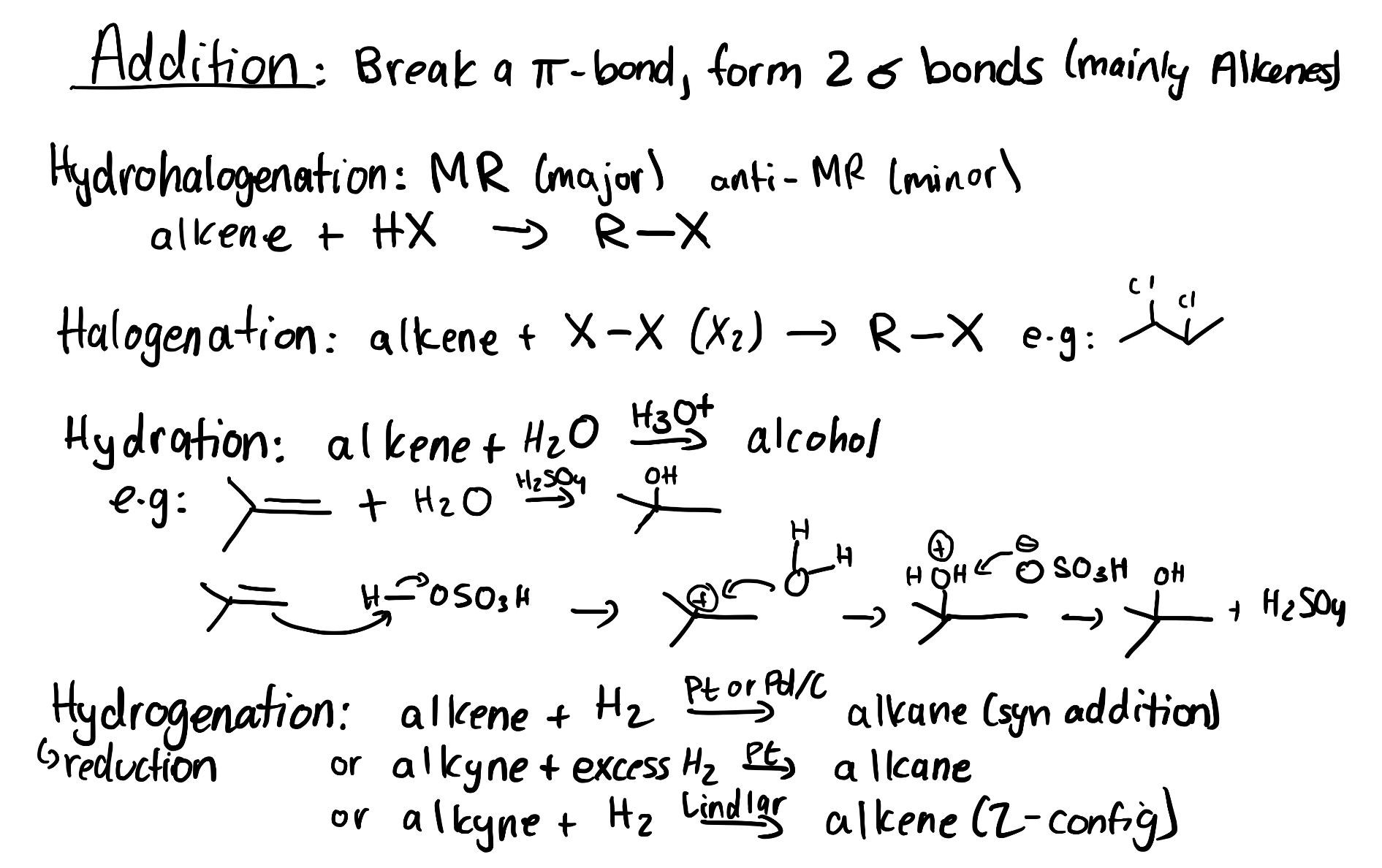
3.2.4 – Halogenation of Alkenes
Halogenation of Alkenes
A halogenation addition reaction occurs between halogens (Br2 and Cl2) and alkenes, creating two adjacent CX bonds, where X is a halogen (Br or Cl). For example, bromination adds one bromine atom to each alkene carbon, resulting in a dibromoalkane.
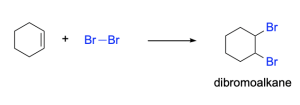
While you do not need to know the mechanism for this reaction (you will see it in year 2 organic chemistry) it’s important to recognize that when two of the same atoms are added across the double bond, Markvonikov’s rule does not apply.
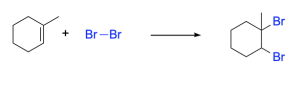
Recall that in addition reactions, one π bond and one σ bond are broken, and two σ bonds are formed. In halogenation of an alkene, the C=C π bond and the X–X bond (where X is a halogen) are broken, while two new C–X σ bonds are formed. In addition, the hybridization at carbon changes: the C=C double bond of reactants have sp2 hybridized carbon atoms, which become sp3 hybridized in the products.
The following video includes a worked example from a previous CHEM 1AA3 test or exam that students struggled with involving alkene reactions. Try solving it on your own before looking at the solution.
(The full solution to this problem can be found in Chapter 5.2)
Study Notes – Chapter 3.2
Diversity in Chemistry: Mario Molina
 Although we go into detail about the halogenation of alkenes to form multi-halogenated molecules, in reality, halogenated compounds pose a huge environmental threat and must be carefully handled and disposed of. Before their effects were known, compounds such as chloroflurocarbon gases were commonly used in refrigerants, aerosol sprays, and in the process of making plastic foam. Mario Molina was the first to discover their toxic effects on the ozone layer, which is crucial for shielding the earth from the sun’s ultraviolet radiation. These gases, when hit with ultraviolet radiation, would release Cl free radicals, which have an unpaired electron and makes them extremelyreactive. These would then interact with molecules of ozone (O3) continuously, starting a chain reaction, resulting in the depletion of the layer ozone molecules. Due to his discovery, Molina became the first scientist of Mexican descent to be awarded a Nobel Prize in Chemistry in 1995 and also obtained the Presidential Medal of Freedom award from President Barack Obama himself in 2013. More information of Mario Molina can be found at the Science History Institute website.
Although we go into detail about the halogenation of alkenes to form multi-halogenated molecules, in reality, halogenated compounds pose a huge environmental threat and must be carefully handled and disposed of. Before their effects were known, compounds such as chloroflurocarbon gases were commonly used in refrigerants, aerosol sprays, and in the process of making plastic foam. Mario Molina was the first to discover their toxic effects on the ozone layer, which is crucial for shielding the earth from the sun’s ultraviolet radiation. These gases, when hit with ultraviolet radiation, would release Cl free radicals, which have an unpaired electron and makes them extremelyreactive. These would then interact with molecules of ozone (O3) continuously, starting a chain reaction, resulting in the depletion of the layer ozone molecules. Due to his discovery, Molina became the first scientist of Mexican descent to be awarded a Nobel Prize in Chemistry in 1995 and also obtained the Presidential Medal of Freedom award from President Barack Obama himself in 2013. More information of Mario Molina can be found at the Science History Institute website.

