3.2.3 – Hydrogenation of Alkenes
Hydrogenation (Reduction) of Alkenes
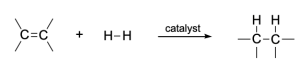
When alkenes react with hydrogen gas in the presence of a variety of metal catalysts, a hydrogen molecule will be added to the double bond in a way that each carbon atom bonds with one hydrogen atom. Such an addition reaction is called hydrogenation (Figure 3.2.3.a).
Recall that in addition reactions, one π bond and one σ bond are broken, and two σ bonds are formed. In hydrogenation of an alkene, the C=C π bond and the H–H bond are broken, while two new C–H σ-bonds are formed. In addition, the hybridization at carbon changes: the C=C double bond of reactants have sp2 hybridized carbon atoms, which become sp3 hybridized in the products.
Catalysts are required for hydrogenation of alkenes, so the reaction can also be called catalytic hydrogenation. Commonly applied metal catalysts include “noble transition metals” like nickel, palladium, and platinum (group 10 metals).
Are You Wondering? The Meaning of Pd/C
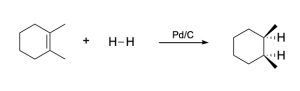
You may see various catalysts written above the arrow in hydrogenation of alkenes. Pd/C is a common catalyst used in hydrogenation, where palladium metal (Pd) is embedded in charcoal (source of carbon atoms). Since the catalyst is heterogenous, its catalytic activity depends on his surface area. Charcoal is a highly porous solid, giving it a high surface area : volume ratio and making it an ideal candidate for heterogenous catalysis. It is also very inexpensive compared to the group 10 metals used in catalytic hydrogenation. The charcoal is embedded with small amounts of Pd (or Pt or Ni) to allow for heterogenous catalytic hydrogenation to occur.
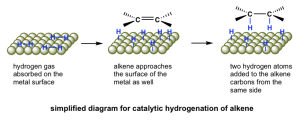
Hydrogenation of an alkene has a high activation energy and will not take place at room temperature without a catalyst present. The catalyst lowers the activation energy by weakening the H-H bond, which makes the reaction feasible at room temperature. The explicit details of the mechanism of catalytic hydrogenation are not completely understood. What is known is that the reaction occurs on the surface of the metal catalyst, and involves both the adsorption of hydrogen gas and the alkene to the metal surface. The metal catalyst also facilitates the breaking of the H2 σ bond and delivery of the hydrogen atoms across the π bond of the alkene. Since this reaction happens on the metal surface, it is observed that both hydrogen atoms are added to the same planar face of the alkene, which we describe as syn addition (Figure 3.2.3.c).
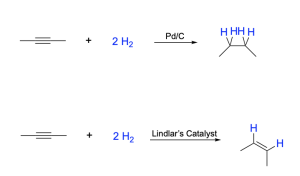
Hydrogenation can also be performed on alkynes. An alkyne contains one σ-bond and two π-bonds. Either one or both π-bonds can undergo hydrogenation via a similar mechanism, to produce an alkene or an alkane, respectively. When adding an excess of hydrogen gas in the presence of a catalyst, the alkyne is fully reduced to an alkane (sp to sp3 hybridization): two π-bonds are broken and four σ bonds (C–H) bonds are formed, with each π bond having hydrogen added across it in a sequential manner (Figure 3.2.3.d, top).
To halt the sequence after just a single addition of hydrogen and obtain an alkene from an alkyne, an alternative catalyst can be used. Lindlar’s catalyst is a poisoned catalyst that will only allow partial reduction to produce an alkene, rather than an alkane. Lindlar’s catalyst still utilizes Pd to facilitate syn addition of hydrogen to one face of the alkyne, but additives are introduced to ‘poison’ its function and prevent further addition of hydrogen across the alkene π bond. Because syn addition adds the hydrogens on the same face of the alkyne, only the Z-isomer (cis-alkene) is produced (Figure 3.2.3.d, bottom).
(The full solution to this problem can be found in Chapter 5.2)
Key Takeaways
- Hydrogenation reactions involve the addition of hydrogen across a double or triple bond, resulting in either an alkane or alkene as products
- The reactants are either an alkene or alkyne, hydrogen gas, and a metal catalyst
- The metal catalyst is Pd/C (palladium and carbon)
- The mechanism involves the absorption of hydrogen on a metal surface, then the hydrogens are added onto the alkene on the same side
- This kind of “same side” addition is known as syn addition
- An alternative catalyst can be used in the reaction of an alkyne to an alkene, known as Lindlar’s catalyst, which is a poisoned (or weaker) catalyst
- In this reaction, because of syn addition, a cis–alkene is the only possible product
Any feedback or comments on this chapter? You may either email chemoer@mcmaster.ca, access this MS Form, or provide a comment in the feedback box below.

