2.5.1 – Alkene Structure
Chapter 2.3 introduced the various functional groups commonly seen in organic molecules. Each functional group has distinct properties that affects the molecule’s reactivity. This chapter will focus on the structure, properties, stereochemistry, and nomenclature of alkenes. Chapter 3.2 will cover the reactivity of alkenes.
Alkene Structure
Molecules are in constant motion, including translation through space, bond vibration, and molecular rotation. Rotation about a carbon-carbon single bond changes the 3-dimensional arrangement of atoms of a molecule. These different arrangement of atoms are called conformations. In Figure 2.5.1.a, two different conformations of butane (an alkane) are shown on the left. These conformations represent rotation about the central carbon-carbon single bond. They are the same molecule and are named the same way: butane.
In contrast, two different structures are shown for the alkene but-2-ene (Figure 2.5.1.a, right). They are different configurations of the molecule. A configuration is a permanent geometry of a molecule resulting from spatial arrangements of its bonds. The two configurations of but-2-ene are different molecules and are named differently: trans–but–2–ene vs cis–but–2–ene.
The cis configuration means that similar substituents are pointing in the same direction on either side of the double bond. For example, in cis–but–2–ene, the methyl groups on either side of the double bond are both pointing up and the implied hydrogens on either side of the double bond are both are pointing down. In contrast, the trans configuration means that similar substituents are pointing in opposite directions on either side of the double bond. For example, in trans–but–2–tene, one methyl group is pointing up and the other is pointing down on either side of the double bond. This nomenclature is further explored in Chapter 2.5.2. You will also see cis and trans terminology in future chemistry and biochemistry courses to designate relationships between different substituents in a molecule.
Although similar, configurations (shown for but-2-ene on the right) and conformations (shown for butane on the left) have different meanings when applied to molecules. This is because molecules with single bonds can rotate freely, but molecules with double bonds (or triple bonds) exhibit restricted rotation.
The reason for restricted rotation in alkenes relates to the orbitals discussed in Chapter 2.2. Carbon atoms in alkenes are sp2 hybridized, meaning they contain three hybridized sp2 orbitals and one unhybridized p-orbital. This unhybridized p-orbital can make a π-bond by overlapping with another p-orbital. The two unhybridized p-orbitals overlap above and below the plane of the molecule (Figure 2.5.1.b). If one of the carbon atoms in the π-bond were to rotate, the p-orbitals would no longer overlap above and below the plane of the molecule, which would break the π-bond. Therefore, alkenes have a restricted rotation.
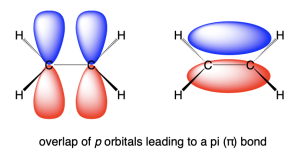
Note the different terminology used for the structural arrangement of alkenes compared to alkanes. Alkenes have different configurations, which are permanent geometries due to restricted rotation about the carbon-carbon double bond. This means that two configurations of an alkene cannot be interconverted, and they are different molecules with different properties. Alkanes have different conformations due to the free rotation about carbon-carbon single bonds. Two conformations of an alkane can be interconverted, which happens regularly at room temperature as molecules are in constant motion.
Impact of Geometry on Melting Point
The cis and trans configurations of alkenes are seen in fatty acids such as oleic and elaidic acid pictured below in Figure 2.5.1.c.
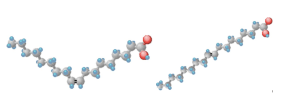
The cis configuration of this molecule (left) creates kinks in the chain, while the trans configuration (right) has a similar zig-zag structure as a saturated hydrocarbon. The kinks in oleic acid prevent molecules from packing tightly together. The lower packing efficiency prevents intermolecular forces of attraction between chains, and thus requires less energy to melt. In contrast, the trans configuration will pack together more efficiently. Greater possibilities for intermolecular attractive forces in elaidic acid means that more energy is required to melt it, raising the melting point compared to the cis configuration. This can be seen in the melting points, where oleic acid has a melting point of 17oC and elaidic acid has one of 52oC.
(The full solution to this problem can be found in Chapter 5.1).
Key Takeaways
- Conformations of molecules are the different non-permanent orientations in three-dimensional space which a molecule can take. This is possible as bonds can rotate, changing the 3D shape.
- This is seen in alkanes, which switch between conformations.
- Configurations are permanent geometric spatial arrangements that a molecule has, for example in alkenes. Confirmations occur in alkenes with cis and trans configurations, being permanent spatial arrangements as the double bond cannot rotate.
- The double bond cannot rotate since the π bond with the p orbitals in alkenes would have to break, requiring energy.
- Alkenes have multiple names for their configurations, such as the cis/trans naming convention
- Cis alkenes have their alkyl chains on the same side of the double bond, while trans alkenes have their alkyl chains on opposite sides of the double bond.
- The properties of cis and trans alkenes vary.
- Trans alkenes have a zig-zag structure that can pack closely together, resulting more intermolecular interactions and higher melting points.
- As cis alkenes have kinks in their chain, they cannot pack together as efficiently, resulting in less intermolecular interactions and lower melting points.
Different 3-dimensional spatial arrangements which atoms in a molecule can freely change between
A permanent geometry of a molecule resulting from spatial arrangements of its bonds, usually seen in alkenes.

