2.4 – IUPAC Nomenclature
Introduction
Chapter 2.1.1. introduced line-angle drawings to draw organic compounds. The ability to draw and recognize these structures is an important skill, as is the ability to name these structures. The International Union of Applied and Pure Chemistry (IUPAC) has standardized the naming of organic compounds by creating a list of rules and regulations to abide by.
This chapter will introduce how to name organic compounds using IUPAC rules.
Prefixes, Parent Chains and Suffixes
When naming an organic structure, or drawing an organic structure based on its name, look for these three key details:
- The suffix indicates the functional group.
- The parent chain indicates the number of carbon atoms in the longest chain that contains the highest priority functional group.
- The prefix indicates the identity and location of substituents on the parent chain. A substituent is an atom or a group of atoms that is attached to the parent chain.
Once these details have been identified, the name of the compound is constructed as follows:
Prefix + parent + suffix
Identifying the functional groups and priority
When naming organic molecules, identify the functional groups first, as this will aid in determining the parent chain. For compounds with more than one functional group, the highest priority functional group defines the final suffix of the parent chain. Lower priority functional groups are considered substituents and are named as prefixes. Figure 2.4.a. shows functional groups listed in priority sequence. Table 2.4.a. shows the name of the functional group when used as a prefix or as a suffix.

Table 2.4.a. Functional groups and their IUPAC prefixes and suffixes.
| Functional Group | Structure | Prefix | Suffix |
| fluorine | fluoro– | None | |
| chlorine | chloro– | None | |
| bromine | bromo– | None | |
| iodine | iodo– | None | |
| ether | 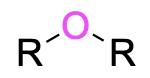 |
alkoxy– | –ether |
| benzene | 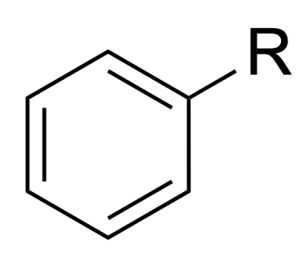 |
phenyl– | –benzene |
| alkane | 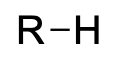 |
alkyl– | –ane |
| alkene | 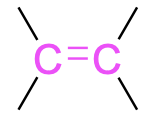 |
alk–#–en–yl– | –ene |
| alkyne | 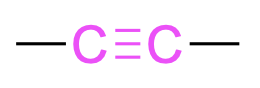 |
alk–#–yn–yl– | –yne |
| alcohol |  |
hydroxy– | –ol |
| aldehyde | 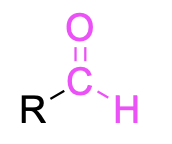 |
None | –al |
| ketone | 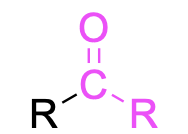 |
None | –one |
| carboxylic acid | 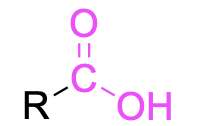 |
None | –oic acid |
| ester | 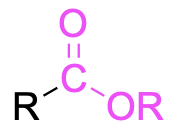 |
None | –oate |
| Primary amine | 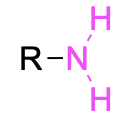 |
None | –amine |
| Secondary amine | 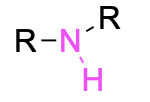 |
None | –amine |
| Tertiary amine | 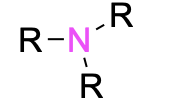 |
None | –amine |
| Primary amide | 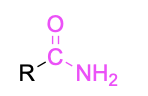 |
None | –amide |
| Secondary amide | 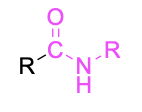 |
None | –amide |
| Tertiary amide | 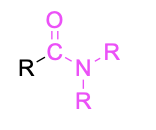 |
None | –amide |
An example of determining priority is shown below in Figure 2.4.b. There are two functional groups present: alcohol and aldehyde. Figure 2.4.a. shows that aldehydes have a higher priority than alcohols, so the aldehyde will be the final suffix (name will end with “–al”), while the alcohol will be a substituent and use the prefix “hydroxy–”.
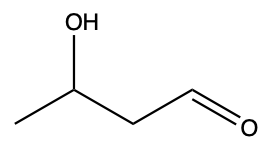
(The full solution to this problem can be found in Chapter 5.1).
The Parent Chain
Once the functional groups have been identified and priority has been assigned, the next step is to identify the parent chain. The parent chain is the carbon chain that contains the highest priority functional group and the greatest number of carbon atoms.
For example, the molecule shown in Figure 2.4.c. is an alkane. To name this molecule, we must identify the chain containing the greatest number of carbon atoms. The parent chain is the path shown in red path, as it contains the most carbon atoms: seven. In comparison, the blue path contains six carbon atoms, and should not be chosen as the parent chain.
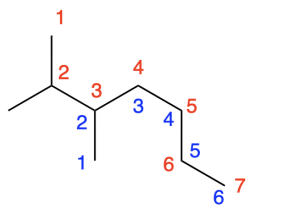
The name of the parent chain depends on the number of carbon atoms. Table 2.4.b below summarizes parent chains containing up to 10 carbons.
Table 2.4.b. Naming parent chains and alkyl substituents containing 1-10 carbons.
| Parent Alkane Name | Alkyl Substituents | Number of Carbon Atoms |
| methane | methyl | 1 |
| ethane | ethyl | 2 |
| propane | propyl | 3 |
| butane | butyl | 4 |
| pentane | pentyl | 5 |
| hexane | hexyl | 6 |
| heptane | heptyl | 7 |
| octane | octyl | 8 |
| nonane | nonyl | 9 |
| decane | decyl | 10 |
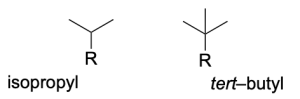
Note that there are common propyl and butyl branched substituents named “isopropyl” and “tert–butyl” respectively, which are pictured below. You will see these substituents often in this course and in second year, so it’s important to become familiar with their structure.
The molecule shown below in Figure 2.4.e is named 2,3-dimethylheptane. In the name, heptane comes from the fact that there are seven carbon atoms in the parent chain (hept) and it is an alkane (suffix –ane). We will examine the prefix (2,3-dimethyl–) in the next section.
Branch Points
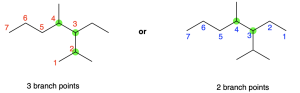
The parent chain may not include all carbon atoms in the molecule. For example, in Figure 2.4.e, the parent chain (shown in red) contains two branch points, at positions 2 and 3 in the chain. A branch point refers to a point where a substituent diverges from the parent chain.
If a compound contains two chains of equal length, the parent chain is designated to be the chain with more branch points. For example, for the molecule shown in Figure 2.4.e, the red path and the blue path both have seven carbon atoms, but the red path has three branch points while the blue path has two branch points (shown in green). The red path should therefore be chosen as the parent chain.
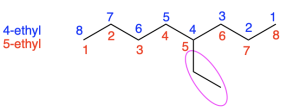
When deciding which end of the parent chain to begin numbering, first choose the path that gives the highest priority functional group the lowest number. If both paths give the same numbering to the highest priority functional group, or the priority of all side chains/functional groups are the same, then choose the path that reaches a branch point sooner. For example, for the molecule shown in Figure 2.4.f, the red path and the blue path both have eight carbon atoms and one branching point. To number the parent chain correctly, the blue path should be chosen because the branch point occurs sooner, at position 4. In comparison, the red path has the first branch point at position 5.
Naming the Branches
To name alkyl branches attached to the parent chain, use the prefix associated with the carbon length (Table 2.4.b), followed by –yl. The position of the branch should be indicated before the branch name with a hyphen separating the number and branch name.
For example, the molecule shown earlier in Figure 2.4.f has one substituent with two carbons. The substituent is named “ethyl”, and we specify that it occurs at position 4 in the parent chain by writing “4-ethyl”. Therefore, the compound shown in Figure 2.4.f is named 4-ethyloctane.
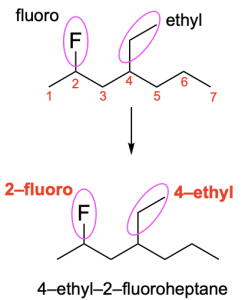
If there is more than one substituent on the parent chain, then the substituents are listed in alphabetical order. For example, the molecule in Figure 2.4.g has two substituents: a fluoro group at position 2 and an ethyl group at position 4 of the parent chain. The word ethyl comes earlier in the alphabet than the word fluoro, and therefore the ethyl group is listed first in the prefixes. There molecule is an alkane with seven carbon atoms in the parent chain, so it is called heptane. Thus, this compound is named 4-ethyl-2-fluoroheptane.
If a molecule has more than one identical substituent, then the prefixes di-, tri-, tetra-, etc., are used to indicate how many identical substituents there are, with the locations on the parent chain separated by commas. For example, let’s once again consider the molecule shown in Figure 2.4.c.
- The molecule is an alkane (-ane).
- There are seven carbon atoms in the parent chain (hept).
- There are two branching points at position 2 and 3, both containing methyl substituents (2,3-dimethyl-).
Therefore, this molecule is named 2,3-dimethylheptane.
A prefix that indicates the quantity of identical substituents (di-, tri-, tetra-, etc.) is not included in alphabetizing the substituents. For example, consider the molecule shown in Figure 2.4.e.
- The molecule is an alkane (-ane).
- There are seven carbon atoms in the parent chain (hept).
- There are three branching points: positions 2 and 4 contain methyl groups (2,4-dimethyl-) while position 3 contains an ethyl group (3-ethyl-).
- Note that ethyl comes first alphabetically compared to methyl; the prefix di- in front of methyl does not count (3-ethyl-2,4-dimethyl-).
Therefore, this molecule is named 3-ethyl-2,4-dimethylheptane.
Combining the Parent Chain and Substituents
When combining the branches and the parent chain to fully name the molecule, write the name as one word, with hyphens separating prefixes, and commas separating numbers. List all substituents in alphabetical order (without alphabetizing prefixes like di, tri, tetra, etc.).
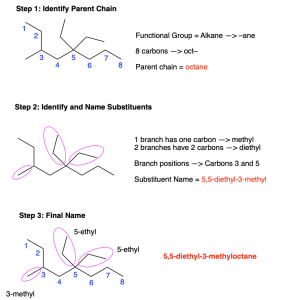
Figure 2.4.h. below illustrates these conventions using the molecule 5,5-diethyl-3-methyloctane.
- The molecule is an alkane (-ane)
- There are eight carbon atoms in the parent chain (oct)
- There are three branching points: position 3 contains a methyl group (3-methyl-) while position 5 contains two ethyl groups (5,5-diethyl-).
- Note that ethyl comes first alphabetically compared to methyl; the prefix di- in front of ethyl does not count when alphabetizing (5,5-diethyl-3-methyl-).
Therefore, this molecule is named 5,5-diethyl-3-methyloctane.
When determining the direction to begin numbering the parent chain, if both paths contain branches at the same number, alphabetization is used to determine the lowest number.
An example is seen below in Figure 2.4.i. Both the red and blue path contain branch points at the same positions (4 and 6). In the blue path, carbon 4 contains a propyl group while carbon 6 contains an ethyl group. In the red path, carbon 4 contains an ethyl group while carbon 6 contains a propyl group. Since the red path contains an ethyl group on the first branch, the red path should be chosen takes priority, as the letter “E” comes first alphabetically.
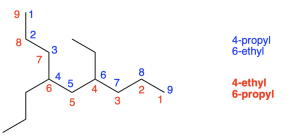
Table 2.4.a mentioned the suffixes and prefixes used for naming the various functional groups. Esters are a special case of combining the parent chain and substituents using the suffixes and prefixes, as shown in the following example (Figure 2.4.j).
(The full solution to this problem can be found in Chapter 5.1).
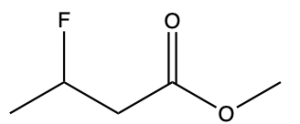
To name esters, split the molecule into two portions (Figure 2.4.k): the portion containing the carbonyl (C=O) group, shown in green, and the substituent that is singly bonded to oxygen, shown in blue. The blue portion is named first, like a prefix, and the green portion is named last.
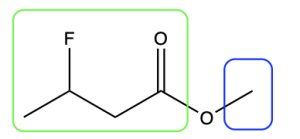
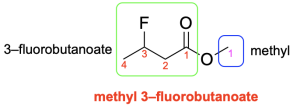
The first step is to name the group singly bonded to oxygen (in blue). In this example, there is one carbon atom (methyl). The next step is to name the portion containing the carbonyl group (in green). Begin numbering this portion starting at the carbonyl carbon atom (this is carbon 1). At carbon 3, there is a fluorine atom (3-fluoro- prefix). The parent chain is 4 carbons long, derived from butane, while the suffix for esters is “-oate” (butanoate). Note that the two portions that comprise the ester (methyl and 3-fluorobutanoate) are separated by a space rather than a hyphen. Therefore, the final name is methyl 3-fluorobutanoate (Figure 2.4.l).
Naming Rings
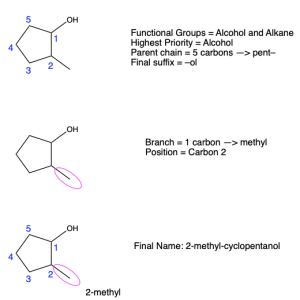
To name the parent chain for cyclic compounds, indicate the number of carbon atoms in the ring, and add the prefix “cyclo”. If there is more than one substituent attached to the ring, number the ring in such a way that the lowest numbered substituent has the highest priority, and continue numbering the ring so that the substituents have the lowest possible number. An example is seen in Figure 2.4.m. There are two functional groups on the cyclopentane ring: an alcohol and an alkyl group. Alcohols have a higher priority, so the ring is labeled starting with carbon 1 at the alcohol, while the methyl group is at carbon 2.
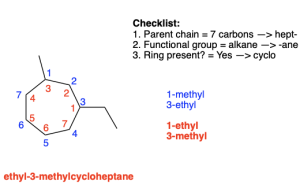
If the substituents have equal priority, then alphabetization is used to determine the numbering scheme. Note that if a substituent is not numbered, it is implied to be at position 1. Figure 2.4.n shows both conventions. The only functional group present is an alkane, so alphabetization is used to determine numbering. The letter “E” comes before the letter “M” in the alphabet, so the numbering will begin with the ethyl group at position 1. The compound can be named 1-ethyl-3-methylcycloheptane or ethyl-3-methylcycloheptane, as the lack of a number before a substituent suggests that it is at position 1.
Converting from Name to Structure
Throughout this chapter, we’ve discussed how to name organic molecules based on their structure. It is also important to be able to recognize and draw the organic molecules from their IUPAC name.
One example is to draw the structure that represents the name 5-fluoro-6,7-dimethyloctan-2-one. The first step is to look at the final suffix and identify the functional group, as that indicates the parent chain. In this example, the final suffix is “–one”. This corresponds to a ketone being the highest priority functional group in the parent chain.
Next, look for the number of carbons in the parent chain, which is located directly before the final suffix. In this example, “octan”, indicates that 8 carbons make up the parent chain. The number 2 in front of “one” denotes the position of that functional group (a ketone) in the parent chain. Therefore, the 8-carbon parent chain has a ketone at position 2 (Figure 2.4.o).
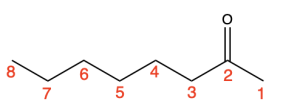
Next, identify the remaining substituents. One substituent listed is “5-fluoro”, meaning there is a fluorine substituent at position 5 of the parent chain. Other substituents listed are “6,7-dimethyl”, so there are 2 methyl groups on positions 6 and 7 of the parent chain (Figure 2.4.p).
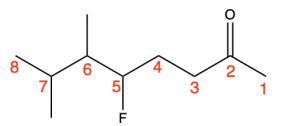
(The full solution to this problem can be found in Chapter 5.1).
Challenging Example
Using the rules from this past chapter, we will work through the example below (Figure 2.4.q).
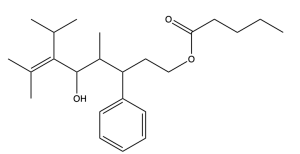
The first step is to identify the functional groups and parent chain (Figure 2.4.r). This molecule contains an ester, alcohol, phenyl group, and alkene. The ester is the highest priority functional group, so it will determine the final suffix. The portion containing the carbonyl (C=O) group (numbered in blue) contains 5 carbons, so the name of the molecule will end in “pentanoate”. The substituent that is singly bonded to oxygen contains 9 carbons in its longest chain (numbered in red), so the prefix will be “non–”. Every other substituent present will be treated as a branch, and the position of the alkene will be specified.
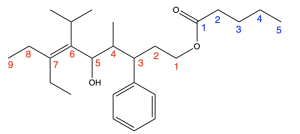
After identifying the parent chain, look for the branches. In this molecule, there are no branches in the carbonyl-containing portion, and five branches in the substituent singly bonded to oxygen: 3-phenyl, 4-methyl, 5-hydroxy, 6-isopropyl and 7-ethyl. In alphabetical order, these prefixes are: 7-ethyl-5-hydroxy-6-isopropyl-4-methyl-3-phenyl.
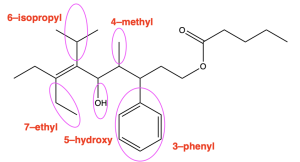
Also note the alkene present between carbon 6 and 7. Functional groups that involve two carbon atoms (such as alkenes and alkynes) are identified by the lower numbered carbon atom, in this case carbon 6. The chain that is singly bonded to the oxygen group contains 9 carbons (nonane), with an alkene at position 6 (6-ene), and is bonded to the carbonyl group at position 1 of the chain (1-yl). Therefore, it is named “non-6-en-1-yl” (without considering the branches in this chain).
For the substituent singly bonded to oxygen, combining all aspects leads to the name: 7-ethyl-5-hydroxy-6-isopropyl-4-methyl-3-phenylnon-6-en-1-yl.
The final step is putting the name together. Remember that branches are placed as prefixes before the parent chain, and the portion that is singly bonded to oxygen is named before the carbonyl-containing portion, with a space between each portion. Therefore, the final name of the molecule would be: 7-ethyl-5-hydroxy-6-isopropyl-4-methyl-3-phenylnon-6-en-1-yl pentanoate.
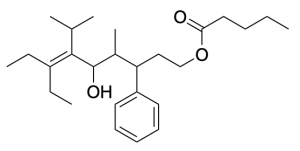
(Click here for full solution).
Key Takeaways
Below is a checklist to ensure you have correctly named the organic molecule:
- Have you identified all functional groups in the molecule?
- Have you assigned priority to the functional groups using Figure 2.4.a. as a guide?
- Have you identified the parent chain?
→ Remember: to identify the parent chain, choose the path that contains more branch points and follows alphabetization if applicable. - Have you identified and named the branches?
- Have you placed the branches in alphabetical order (not including di-, tri-, etc.)?
- Is the molecule a ring?
→ If so, include the prefix “cyclo–” - Have you put the names of the branches and parent chain together to yield the final name, with the branches first and the parent chain last?

