2.3 – Functional Groups
What are Functional Groups?
A functional group is a group of atoms bonded together in a particular way that impact the molecule’s chemical behaviour. Molecules with the same functional group can typically undergo the same chemical reactions. The greater the number of functional groups, the greater the diversity of chemical reactions the molecule can undergo. Being able to recognize a molecule’s functional groups will help you to understand and predict its reactivity.
Examples of functional groups are shown in Figure 2.3.a.
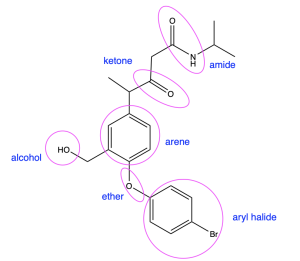
Classes of Organic Compounds and Their Functional Groups
The table below summarizes the structures of various functional groups that you must be able to recognize. Areas highlighted in pink are the functional groups of the molecule.
Table 2.3.a. Common functional groups in organic chemistry.
| Class | General Structural Formula | Example |
| Alkane | 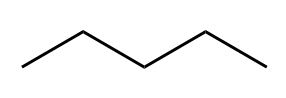 |
|
| Alkene | 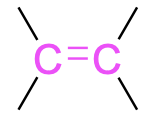 |
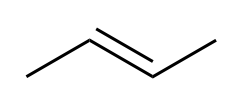 |
| Alkyne | 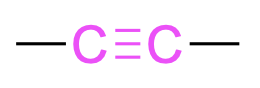 |
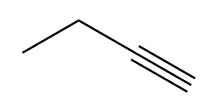 |
| Alcohol |  |
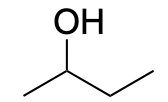 |
| Alkyl halide | 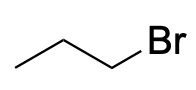 |
|
| Ether | 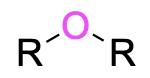 |
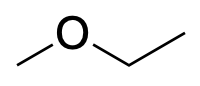 |
| Primary amine* | 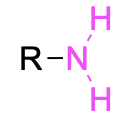 |
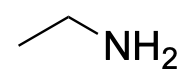 |
| Secondary amine* | 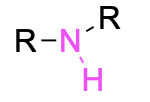 |
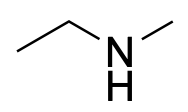 |
| Tertiary amine* | 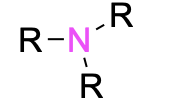 |
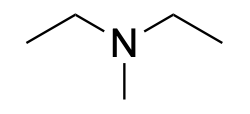 |
| Thiol | 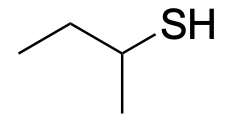 |
|
| Aldehyde | 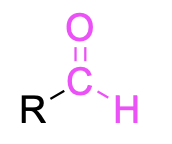 |
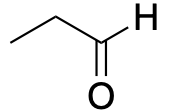 |
| Ketone | 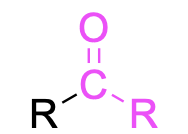 |
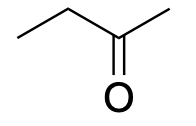 |
| Carboxylic acid | 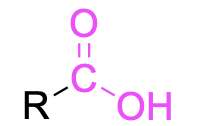 |
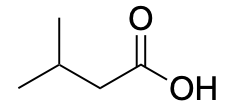 |
| Ester | 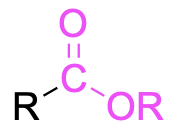 |
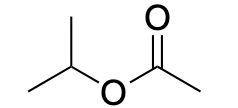 |
| Carboxylic acid anhydride | 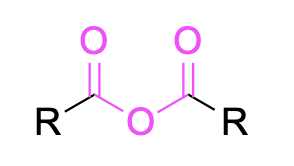 |
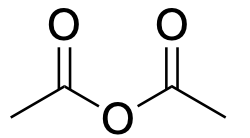 |
| Acid halide | 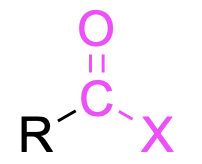 |
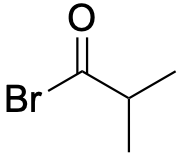 |
| Primary amide* | 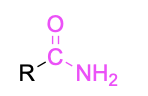 |
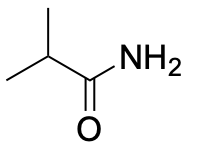 |
| Secondary amide* | 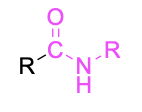 |
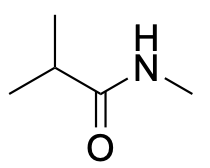 |
| Tertiary amide* | 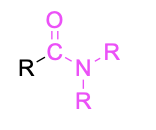 |
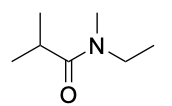 |
| Arene | 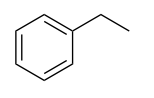 |
|
| Aryl halide | 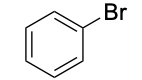 |
|
| Phenol | 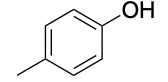 |
*For more information about how amines and amides can be designated as primary, secondary, and tertiary, see the Are You Wondering box at the end of this chapter.
(The full solution to this problem can be found in Chapter 5.1).
Primary, Secondary, Tertiary and Quaternary Designation
To predict a molecule’s reactivity (which we will see in Chapter 3), it is important to identify not only its functional groups but also the number of substituents bonded to a particular atom.
A saturated carbon is a carbon atom that is bonded to other atoms through single bonds only. Saturated carbon atoms can be designated as either methyl, 1°, 2°, 3° or 4° depending on how many other carbon atoms are bonded to it.
If a carbon atom is bonded to one carbon substituent, it is said to be primary.
If a carbon atom is bonded to two carbon substituents, it is said to be secondary.
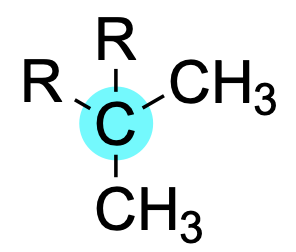
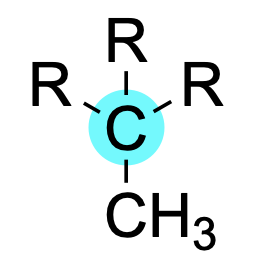
If a carbon atom is bonded to three carbon substituents, it is said to be tertiary.
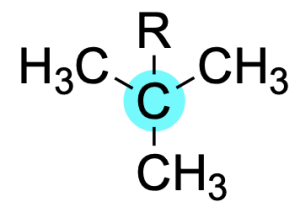
If a carbon atom is bonded to four carbon substituents, it is said to be quaternary.
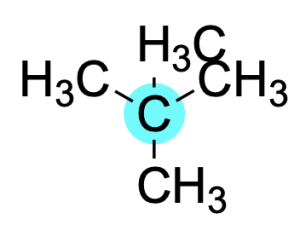
This designation can also be applied to saturated alkyl halides, carbocations, and alcohols. The figure below shows examples of each.
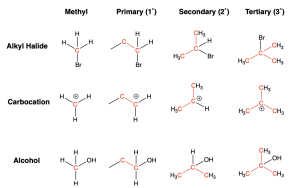
Note that when carbon is bonded to a heteroatom (like a halogen or an oxygen shown in the examples above), there is no quaternary designation. This is because carbon cannot have more than 4 bonds.
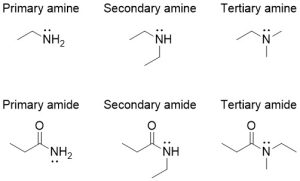
Are You Wondering? Primary, Secondary, and Tertiary Centres
The designation of primary, secondary, and tertiary also applies to the nitrogen centre in amines and amides (Figure 2.3.g). To determine whether the amine or amide is primary, secondary, or tertiary, count the number of carbon-containing substituents bonded to the nitrogen centre. If there is one carbon-containing substituent, then it is a primary amine. If there are two carbon-containing substituents, then it is a secondary amine. If there are three carbon-containing substituents, then it is a tertiary amine.
(The full solution to this problem can be found in Chapter 5.1).
Key Takeaways
- A functional group is defined as two or more bonded atoms which affect a molecules reactivity or chemical properties.
- The same functional group on two different molecules will exhibit similar reactivity
- Carbon bonded to other atoms with single bonds only is called a saturated carbon.
- Carbon can be designated primary, secondary, tertiary or quaternary, depending on how many other carbons it is bound to. Carbon can also be referred to as methane if it is not bound to any other carbons.
- Primary carbon: a carbon bound to one other carbon.
- Secondary carbon: a carbon bound to two other carbons.
- Tertiary carbon: a carbon bound to three other carbons.
- Quaternary carbon: a carbon bound to four other carbons.
- This characterization of carbons can be applied to any other carbon bound to functional groups such as alcohols, alkyl halides, etc.
Key terms in this chapter:
| Key term | Definition |
| Heteroatom | An atom other than carbon, such as oxygen, nitrogen, and sulfur. |
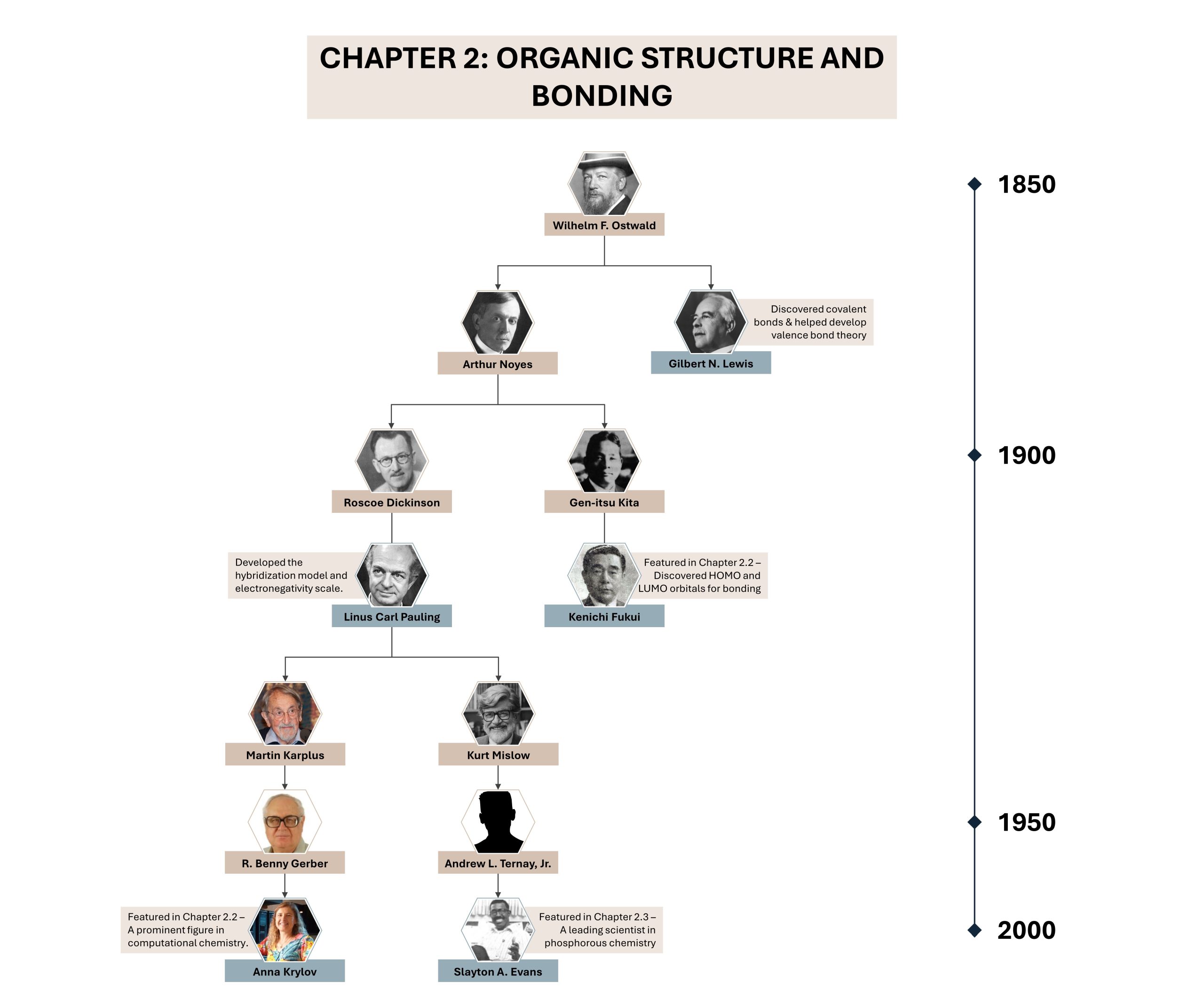
Diversity in Chemistry: Slayton Evans Jr.

Although a wide variety of the most important functional groups have been introduced in this chapter, this does not even cover half of the known groups. Phosphorus, P, is a common heteroatom found in biology that makes up a number of functional groups, such as the phosphate group.Slayton Evans Jr., a professor at the University of North Carolina, was a trailblazer in organophosphorus chemistry, resulting in a deeper understanding of their properties and aiding in the development of novel methods to synthesize them for pharmaceutical use. Raised in Mississippi, Evans lived in a segregated public housing project with his family and became enamoured by science after he received a chemistry lab toy set and miniature microscope.
After his postdoctoral fellowship, Evans joined the University of North Carolina as the first African American chemistry professor at the university in 200 years, and quickly became successful due to his excellent work and dedication to teaching. Evans was also known as an outstanding mentor who advocated for recruiting underprivileged and students from underrepresented groups, closely guiding many of his undergraduate and graduate students. More information on Evans can be found on his page at UNC. Below shows a snapshot of Evans’ academic tree, with connections to other famous scientists such as Linus Carl Pauling, Kenichi Fukui, and Anna Krylov.
A group of atoms bonded together in a particular way that impact the molecule’s chemical behaviour. Examples include alkanes, hydroxyls, and amines.
An atom other than carbon, such as oxygen, nitrogen, and sulfur.

