1.4 Experimental Design and Ethics
LEARNING OBJECTIVES
- Describe different aspects of experimental design.
- Apply ethical behaviour in statistical analysis.
Does aspirin reduce the risk of heart attacks? Is one brand of fertilizer more effective at growing roses than another? Is fatigue as dangerous to a driver as the influence of alcohol? Questions like these are answered using randomized experiments. Proper study design ensures the production of reliable, accurate data.
The purpose of an experiment is to investigate the relationship between two variables. When one variable causes a change in another, we call the first variable the explanatory variable. The affected variable is called the response variable. In a randomized experiment, the researcher manipulates values of the explanatory variable and measures the resulting changes in the response variable. The different values of the explanatory variable are called treatments. An experimental unit is a single object or individual to be measured.
Video: "Observational Studies and Experiments" by ProfessorMcComb [3:06] is licensed under the Standard YouTube License.Transcript and closed captions available on YouTube.
Suppose we want to investigate the effectiveness of vitamin E in preventing disease. We recruit a group of subjects and ask them if they regularly take vitamin E. We notice that the subjects who take vitamin E exhibit better health on average than those who do not. Does this prove that vitamin E is effective in disease prevention? No, it does not. There are many differences between the two groups compared, in addition to vitamin E consumption. People who take vitamin E often take other steps to improve their health, such as exercise, diet, other vitamin supplements, or choosing not to smoke. Any one of these factors could be influencing a person's health. As described, this study does not prove that vitamin E is the key to disease prevention.
Additional variables that can cloud a study are called lurking variables. In order to prove that the explanatory variable is the cause of a change in the response variable, it is necessary to isolate the explanatory variable. The researcher must design their experiment in such a way that there is only one difference between the groups being compared: the planned treatments. This is accomplished by the random assignment of experimental units to treatment groups. When subjects are assigned treatments randomly, all of the potential lurking variables are spread equally among the groups. At this point, the only difference between groups is the one imposed by the researcher. Therefore, different outcomes measured in the response variable must be a direct result of the different treatments. In this way, an experiment can prove a cause-and-effect connection between the explanatory and response variables.
The power of suggestion can have an important influence on the outcome of an experiment. Studies have shown that the expectation of the study participant can be as important as the actual medication. When participation in a study prompts a physical response from a participant, it is difficult to isolate the effects of the explanatory variable. To counter the power of suggestion, researchers set aside one treatment group as a control group. This group is given a placebo treatment—a treatment that cannot influence the response variable. The control group helps researchers balance the effects of being in an experiment with the effects of the active treatments. Of course, if someone is participating in a study and they know that they are receiving a pill which contains no actual medication, then the power of suggestion is no longer a factor. Blinding in a randomized experiment preserves the power of suggestion. When a person involved in a research study is blinded, they do not know who is receiving the active treatment(s) and who is receiving the placebo treatment. A double-blind experiment is one in which both the subjects and the researchers involved with the subjects are blinded.
EXAMPLE
Researchers want to investigate whether taking aspirin regularly reduces the risk of heart attack. Four hundred men between the ages of [latex]50[/latex] and [latex]84[/latex] are recruited as participants. The men are divided randomly into two groups: one group will take aspirin, and the other group will take a placebo. Each man takes one pill each day for three years, but he does not know whether he is taking aspirin or the placebo. At the end of the study, researchers count the number of men in each group who have had heart attacks.
Identify the following values for this study: population, sample, experimental units, explanatory variable, response variable, and treatments.
Solution
- The population is men aged [latex]50[/latex] to [latex]84[/latex].
- The sample is the [latex]400[/latex] men who participated.
- The experimental units are the individual men in the study.
- The explanatory variable is the oral medication.
- The treatments are aspirin and a placebo.
- The response variable is whether a subject had a heart attack.
EXAMPLE
The Smell & Taste Treatment and Research Foundation conducted a study to investigate whether smell can affect learning. Subjects completed mazes multiple times while wearing masks. They completed the pencil and paper mazes three times wearing floral-scented masks and three times with unscented masks. Participants were assigned at random to wear the floral mask during the first three trials or during the last three trials. For each trial, researchers recorded the time it took to complete the maze and the subject’s impression of the mask’s scent: positive, negative, or neutral.
- Describe the explanatory and response variables in this study.
- What are the treatments?
- Identify any lurking variables that could interfere with this study.
- Is it possible to use blinding in this study?
Solution
- The explanatory variable is scent, and the response variable is the time it takes to complete the maze.
- There are two treatments: a floral-scented mask and an unscented mask.
- All subjects experienced both treatments. The order of treatments was randomly assigned, so there were no differences between the treatment groups. Random assignment eliminates the problem of lurking variables.
- Subjects will clearly know whether they can smell flowers or not, so subjects cannot be blinded in this study. However, researchers timing the mazes can be blinded. The researcher who is observing a subject will not know which mask is being worn.
EXAMPLE
A researcher wants to study the effects of birth order on personality. Explain why this study could not be conducted as a randomized experiment. What is the main problem in a study that cannot be designed as a randomized experiment?
Solution
The explanatory variable is birth order. We cannot randomly assign a person’s birth order. Random assignment eliminates the impact of lurking variables. When we cannot assign subjects to treatment groups at random, there will be differences between the groups other than the explanatory variable.
TRY IT
A researcher wants to study the effects of texting on driving performance. The researcher designs a study to test the response time of drivers while texting and while driving only and measures how many seconds it takes for a driver to respond when a leading car hits the brakes?
- Describe the explanatory and response variables in the study.
- What are the treatments?
- What should the researcher consider when selecting participants?
- The researcher considers dividing participants randomly into two groups: one to drive without distraction and one to text and drive simultaneously. Is this a good idea? Why or why not?
- Identify any lurking variables that could interfere with this study.
- How can blinding be used in this study?
Click to see Solution
- The explanatory variable is texting, and the response variable is time to hit the brakes.
- There are two treatments: driving while texting and driving only.
- Participants should be experienced drivers.
- This is not a good idea. The purpose of the study is to test the effect of texting on driving. To draw a meaningful conclusion, each driver must perform the test for both treatments: driving while texting and driving only.
- If each participant experiences both treatments and the treatments are randomly assigned, there are no lurking variables.
- The participants cannot be blinded because they will know if they are texting while driving or driving only. The researcher timing the drivers could potentially be blinded.
Ethics
The widespread misuse and misrepresentation of statistical information often gives the field a bad name. Some say that “numbers don’t lie,” but the people who use numbers to support their claims often do.
A recent investigation of famous social psychologist Diederik Stapel has led to the retraction of his articles from some of the world’s top journals, including the Journal of Experimental Social Psychology, Social Psychology, Basic and Applied Social Psychology, British Journal of Social Psychology, and the magazine Science. Diederik Stapel is a former professor at Tilburg University in the Netherlands. Recently, an extensive investigation involving three universities where Stapel worked concluded that the psychologist is guilty of fraud on a colossal scale. Falsified data taints over 55 papers he authored and 10 Ph.D. dissertations that he supervised.
The committee investigating Stapel concluded that he is guilty of several practices, including:
- creating datasets, which largely confirmed the prior expectations;
- altering data in existing datasets;
- changing measuring instruments without reporting the change and
- misrepresenting the number of experimental subjects.
Clearly, it is never acceptable to falsify data the way this researcher did. Sometimes, however, violations of ethics are not as easy to spot.
Researchers have a responsibility to verify that proper methods are being followed. Many of Stapel’s co-authors should have spotted irregularities in his data. Unfortunately, they did not know very much about statistical analysis, and they simply trusted that he was collecting and reporting data properly.
Many types of statistical fraud are difficult to spot. Some researchers simply stop collecting data once they have just enough to prove what they had hoped to prove. They do not want to take the chance that a more extensive study would complicate their lives by producing data contradicting their hypothesis.
Professional organizations, like the American Statistical Association, clearly define expectations for researchers. There are even laws in the federal code about the use of research data.
When a statistical study uses human participants, as in medical studies, both ethics and the law dictate that researchers should be mindful of the safety of their research subjects. Most countries have federal regulations that protect participants in research studies. When a university or other research institution engages in research, it must ensure the safety of all human subjects. For this reason, research institutions establish oversight committees, commonly known as Institutional Review Boards (IRB). All planned studies must be approved in advance by the IRB. Key protections that are mandated by law include the following:
- Risks to participants must be minimized and reasonable with respect to projected benefits.
- Participants must give informed consent. This means that the risks of participation must be clearly explained to the subjects of the study. Subjects must consent in writing, and researchers are required to keep documentation of their consent.
- Data collected from individuals must be guarded carefully to protect their privacy.
These ideas may seem fundamental, but they can be very difficult to verify in practice. Is removing a participant’s name from the data record sufficient to protect privacy? Perhaps the person’s identity could be discovered from the data that remains. What happens if the study does not proceed as planned and risks arise that were not anticipated? When is informed consent really necessary? Suppose your doctor wants a blood sample to check your cholesterol level. Once the sample has been tested, you expect the lab to dispose of the remaining blood. At that point, the blood becomes biological waste. Does a researcher have the right to take it for use in a study?
It is important that students of statistics take time to consider the ethical questions that arise in statistical studies. How prevalent is fraud in statistical studies? You might be surprised—and disappointed. There is a website dedicated to cataloguing retractions of study articles that have been proven fraudulent. A quick glance will show that the misuse of statistics is a bigger problem than most people realize.
Vigilance against fraud requires knowledge. Learning the basic theory of statistics empowers users and observers to analyze statistical studies critically.
EXAMPLE
Describe the unethical behaviour in each example and describe how it could impact the reliability of the resulting data. Explain how the problem should be corrected.
A researcher is collecting data in a community.
- She selects a block where she is comfortable walking because she knows many of the people living on the street.
- No one seems to be home at four houses on her route. She does not record the addresses and does not return at a later time to try to find residents at home.
- She skips four houses on her route because she is running late for an appointment. When she gets home, she fills in the forms by selecting random answers from other residents in the neighbourhood.
Solution
- By selecting a convenient sample, the researcher is intentionally selecting a sample that could be biased. Claiming that this sample represents the community is misleading. The researcher needs to select areas in the community at random.
- Intentionally omitting relevant data will create bias in the sample. Suppose the researcher is gathering information about jobs and child care. By ignoring people who are not home, she may be missing data from working families that are relevant to her study. She needs to make every effort to interview all members of the target sample.
- It is never acceptable to fake data. Even though the responses she uses are “real” responses provided by other participants, the duplication is fraudulent and can create bias in the data. She needs to work diligently to interview everyone on her route.
TRY IT
Describe the unethical behaviour, if any, in each example and describe how it could impact the reliability of the resulting data. Explain how the problem should be corrected.
A study is commissioned to determine the favourite brand of fruit juice among teens in California.
- The survey is commissioned by the seller of a popular brand of apple juice.
- There are only two types of juice included in the study: apple juice and cranberry juice.
- Researchers allow participants to see the brand of juice as samples are poured for a taste test.
- Twenty-five percent of participants prefer Brand X, 33% prefer Brand Y, and 42% have no preference between the two brands. Brand X references the study in a commercial, saying, “Most teens like Brand X as much as or more than Brand Y.”
Click to see Solution
- The seller of the apple juice has a vested interest in the outcome of the study. Their participation in the study may result in misleading or biased data.
- The study is intentionally omitting relevant data. What if a teen's favourite fruit juice is orange? The study misses data that is relevant to the study.
- Because participants can see the brand, familiarity or preference for one brand over another may influence participants to pick that brand, regardless of which juice flavour they prefer. This introduces bias into the sample.
- The company is making a fraudulent claim about the data in the commercial.
Exercises
- Discuss potential violations of the rule requiring informed consent.
- Inmates in a correctional facility are offered good behaviour credit in return for participation in a study.
- A research study is designed to investigate a new children’s allergy medication.
- Participants in a study are told that the new medication being tested is highly promising, but they are not told that only a small portion of participants will receive the new medication. Others will receive placebo treatments and traditional treatments.
Click to see Answer
- Inmates may not feel comfortable refusing participation or may feel obligated to take advantage of the promised benefits. They may not feel truly free to refuse participation.
- Parents can provide consent on behalf of their children, but children are not competent to provide consent for themselves.
- All risks and benefits must be clearly outlined. Study participants must be informed of relevant aspects of the study in order to give appropriate consent.
- How does sleep deprivation affect your ability to drive? A recent study measured the effects on [latex]19[/latex] professional drivers. Each driver participated in two experimental sessions: one after normal sleep and one after [latex]27[/latex] hours of total sleep deprivation. The treatments were assigned in random order. In each session, performance was measured on a variety of tasks, including a driving simulation. Use key terms from this chapter to describe the design of this experiment.
Click to see Answer
- Explanatory variable: the amount of sleep.
- Response variable: performance measured in assigned tasks.
- Treatments: normal sleep and 27 hours of total sleep deprivation.
- Experimental units: 19 professional drivers.
- Lurking variables: none – all drivers participated in both treatments.
- Random assignment: treatments were assigned in random order; this eliminated the effect of any “learning” that may take place during the first experimental session.
- Control/Placebo: completing the experimental session under normal sleep conditions.
- Blinding: researchers evaluating subjects’ performance must not know which treatment is being applied at the time89. You cannot assume that the numbers of complaints reflect the quality of the airlines. The airlines shown with the greatest number of complaints are the ones with the most passengers. You must consider the appropriateness of methods for presenting data; in this case, displaying totals is misleading.
- An advertisement for Acme Investments displays the two graphs in the figure below to show the value of Acme’s product in comparison with the Other Guy’s product. Describe the potentially misleading visual effect of these comparison graphs. How can this be corrected?
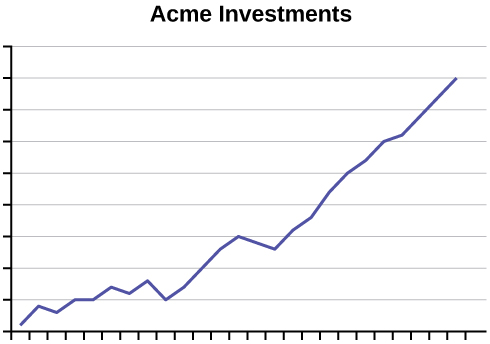
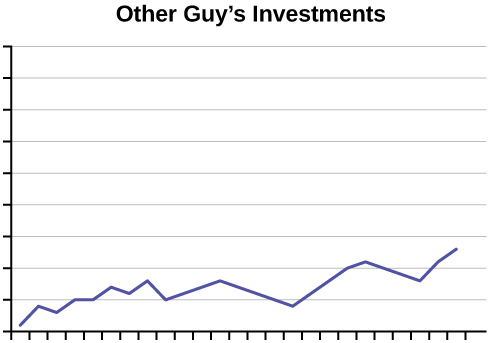
Click to see Answer
There is no scale on either graph, making it impossible to tell which one produced the best return on investments. The results are shown in separate graphs, making it difficult to compare the results directly. To fix this, plot both graphs on the same set of axes and include a scale on the axes.
- The graph in the figure below shows the number of complaints for six different airlines as reported to the US Department of Transportation in February 2013. Alaska, Pinnacle, and Airtran Airlines have far fewer complaints reported than American, Delta, and United. Can we conclude that American, Delta, and United are the worst airline carriers since they have the most complaints?
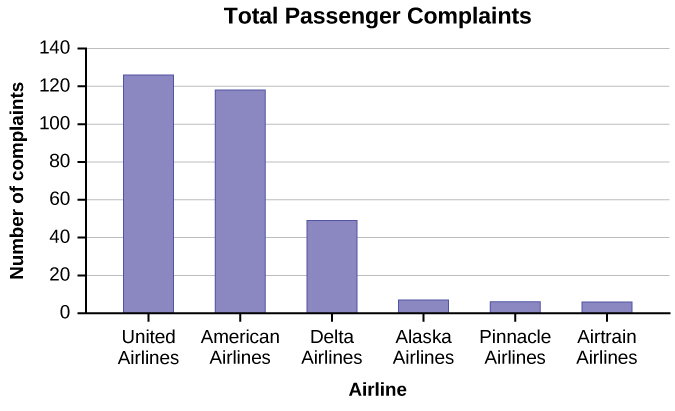
Click to see Answer
Because American, United and Delta are the largest carries, with the largest number of total passengers, and so will have the largest number of complaints. A more accurate graph would be to show the proportion of complaints out of the total number of passengers.
"1.5 Experimental Design and Ethics" and “1.6 Exercises” from Introduction to Statistics by Valerie Watts is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License, except where otherwise noted.

