Learning Objectives
By the end of this section, you will be able to:
The Ovarian Cycle
The ovarian cycle is a set of predictable changes in a female’s oocytes and ovarian follicles. During a woman’s reproductive years, it is a roughly 28-day cycle that can be correlated with, but is not the same as, the menstrual cycle (discussed shortly). The cycle includes two interrelated processes: oogenesis (the production of female gametes) and folliculogenesis (the growth and development of ovarian follicles).
Oogenesis
Gametogenesis in females is called oogenesis. The process begins with the ovarian stem cells, or oogonia (Figure 27.3.1). Oogonia are formed during fetal development, and divide via mitosis, much like spermatogonia in the testis. Unlike spermatogonia, however, oogonia form primary oocytes in the fetal ovary prior to birth. These primary oocytes are then arrested in this stage of meiosis I, only to resume it years later, beginning at puberty and continuing until the woman is near menopause (the cessation of a woman’s reproductive functions). The number of primary oocytes present in the ovaries declines from one to two million in an infant, to approximately 400,000 at puberty, to zero by the end of menopause.
The initiation of ovulation—the release of an oocyte from the ovary—marks the transition from puberty into reproductive maturity for women. From then on, throughout a woman’s reproductive years, ovulation occurs approximately once every 28 days. Just prior to ovulation, a surge of luteinizing hormone triggers the resumption of meiosis in a primary oocyte. This initiates the transition from primary to secondary oocyte. However, as you can see in Figure 27.3.1, this cell division does not result in two identical cells. Instead, the cytoplasm is divided unequally, and one daughter cell is much larger than the other. This larger cell, the secondary oocyte, eventually leaves the ovary during ovulation. The smaller cell, called the first polar body, may or may not complete meiosis and produce second polar bodies; in either case, it eventually disintegrates. Therefore, even though oogenesis produces up to four cells, only one survives.
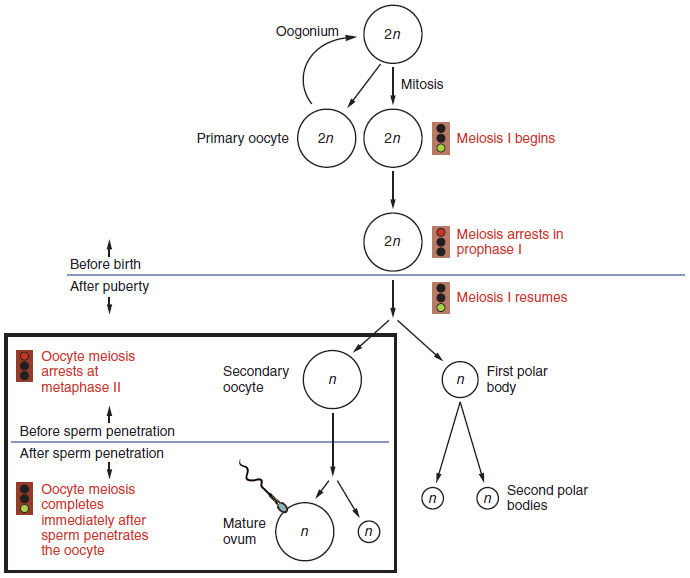
How does the diploid secondary oocyte become an ovum—the haploid female gamete? Meiosis of a secondary oocyte is completed only if a sperm succeeds in penetrating its barriers. Meiosis II then resumes, producing one haploid ovum that, at the instant of fertilization by a (haploid) sperm, becomes the first diploid cell of the new offspring (a zygote). Thus, the ovum can be thought of as a brief, transitional, haploid stage between the diploid oocyte and diploid zygote.
The larger amount of cytoplasm contained in the female gamete is used to supply the developing zygote with nutrients during the period between fertilization and implantation into the uterus. Interestingly, sperm contribute only DNA at fertilization —not cytoplasm. Therefore, the cytoplasm and all of the cytoplasmic organelles in the developing embryo are of maternal origin. This includes mitochondria, which contain their own DNA. Scientific research in the 1980s determined that mitochondrial DNA was maternally inherited, meaning that you can trace your mitochondrial DNA directly to your mother, her mother, and so on back through your female ancestors.
Folliculogenesis
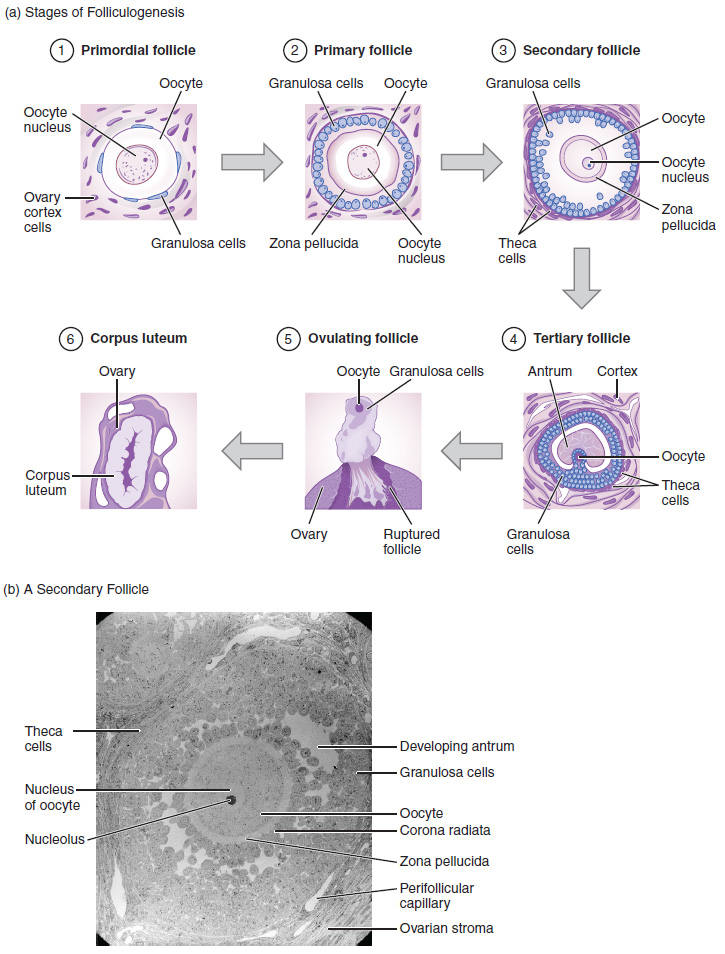
Again, ovarian follicles are oocytes and their supporting cells. They grow and develop in a process called folliculogenesis, which typically leads to ovulation of one follicle approximately every 28 days, along with death to multiple other follicles. The death of ovarian follicles is called atresia, and can occur at any point during follicular development. Recall that, a female infant at birth will have one to two million oocytes within her ovarian follicles, and that this number declines throughout life until menopause, when no follicles remain. As you’ll see next, follicles progress from primordial, to primary, to secondary and tertiary stages prior to ovulation—with the oocyte inside the follicle remaining as a primary oocyte until right before ovulation.
Folliculogenesis begins with follicles in a resting state. These small primordial follicles are present in newborn females and are the prevailing follicle type in the adult ovary (Figure 27.3.2). Primordial follicles have only a single flat layer of support cells, called granulosa cells, that surround the oocyte, and they can stay in this resting state for years—some until right before menopause.
After puberty, a few primordial follicles will respond to a recruitment signal each day, and will join a pool of immature growing follicles called primary follicles. Primary follicles start with a single layer of granulosa cells, but the granulosa cells then become active and transition from a flat or squamous shape to a rounded, cuboidal shape as they increase in size and proliferate. As the granulosa cells divide, the follicles—now called secondary follicles (see Figure 27.3.2)—increase in diameter, adding a new outer layer of connective tissue, blood vessels, and theca cells—cells that work with the granulosa cells to produce estrogens.
Within the growing secondary follicle, the primary oocyte now secretes a thin acellular membrane called the zona pellucida that will play a critical role in fertilization. A thick fluid, called follicular fluid, that has formed between the granulosa cells also begins to collect into one large pool, or antrum. Follicles in which the antrum has become large and fully formed are considered tertiary follicles (or antral follicles). Several follicles reach the tertiary stage at the same time, and most of these will undergo atresia. The one that does not die will continue to grow and develop until ovulation, when it will expel its secondary oocyte surrounded by several layers of granulosa cells from the ovary. Keep in mind that most follicles don’t make it to this point. In fact, roughly 99 percent of the follicles in the ovary will undergo atresia, which can occur at any stage of folliculogenesis.
Hormonal Control of the Ovarian Cycle
The process of development that we have just described, from primordial follicle to early tertiary follicle, takes approximately two months in humans. The final stages of development of a small cohort of tertiary follicles, ending with ovulation of a secondary oocyte, occur over a course of approximately 28 days. These changes are regulated by many of the same hormones that regulate the male reproductive system, including GnRH, LH, and FSH.
As in men, the hypothalamus produces GnRH, a hormone that signals the anterior pituitary gland to produce the gonadotropins FSH and LH (Figure 27.3.3). These gonadotropins leave the pituitary and travel through the bloodstream to the ovaries, where they bind to receptors on the granulosa and theca cells of the follicles. FSH stimulates the follicles to grow (hence its name of follicle-stimulating hormone), and the five or six tertiary follicles expand in diameter. The release of LH also stimulates the granulosa and theca cells of the follicles to produce the sex steroid hormone estradiol, a type of estrogen. This phase of the ovarian cycle, when the tertiary follicles are growing and secreting estrogen, is known as the follicular phase.
The more granulosa and theca cells a follicle has (that is, the larger and more developed it is), the more estrogen it will produce in response to LH stimulation. As a result of these large follicles producing large amounts of estrogen, systemic plasma estrogen concentrations increase. Following a classic negative feedback loop, the high concentrations of estrogen will stimulate the hypothalamus and pituitary to reduce the production of GnRH, LH, and FSH. Because the large tertiary follicles require FSH to grow and survive at this point, this decline in FSH caused by negative feedback leads most of them to die (atresia). Typically only one follicle, now called the dominant follicle, will survive this reduction in FSH, and this follicle will be the one that releases an oocyte. Scientists have studied many factors that lead to a particular follicle becoming dominant: size, the number of granulosa cells, and the number of FSH receptors on those granulosa cells all contribute to a follicle becoming the one surviving dominant follicle.
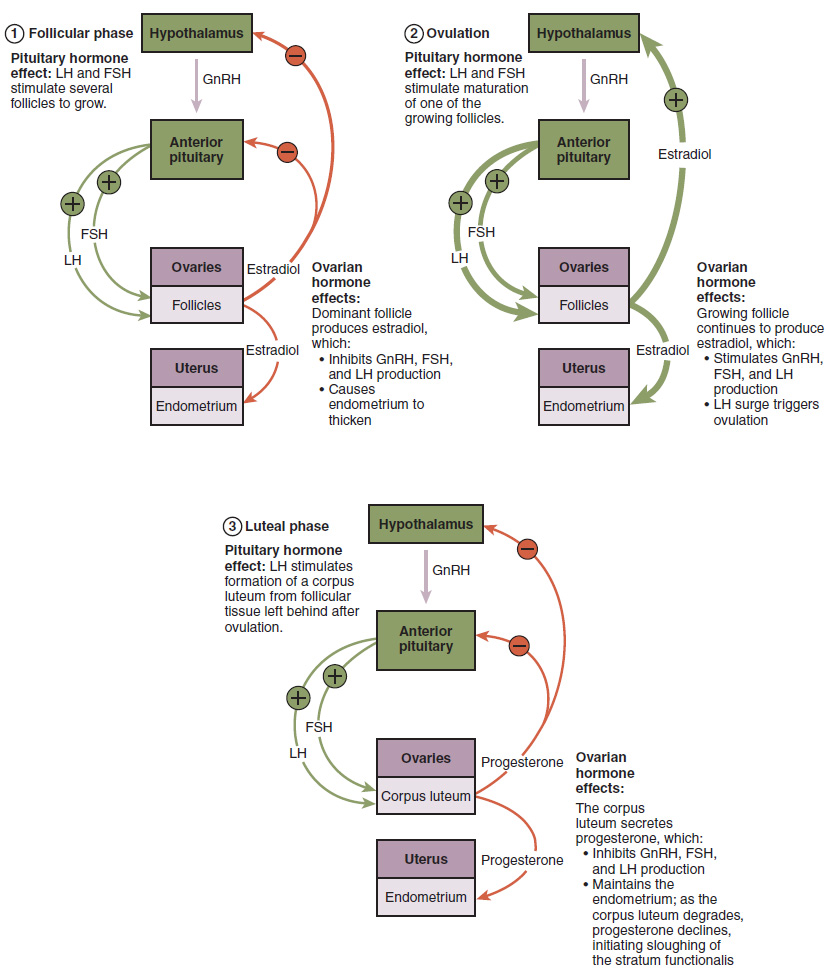
When only the one dominant follicle remains in the ovary, it again begins to secrete estrogen. It produces more estrogen than all of the developing follicles did together before the negative feedback occurred. It produces so much estrogen that the normal negative feedback doesn’t occur. Instead, these extremely high concentrations of systemic plasma estrogen trigger a regulatory switch in the anterior pituitary that responds by secreting large amounts of LH and FSH into the bloodstream (see Figure 27.3.3). The positive feedback loop by which more estrogen triggers release of more LH and FSH only occurs at this point in the cycle.
It is this large burst of LH (called the LH surge) that leads to ovulation of the dominant follicle. The LH surge induces many changes in the dominant follicle, including stimulating the resumption of meiosis of the primary oocyte to a secondary oocyte. As noted earlier, the polar body that results from unequal cell division simply degrades. The LH surge also triggers proteases (enzymes that cleave proteins) to break down structural proteins in the ovary wall on the surface of the bulging dominant follicle. This degradation of the wall, combined with pressure from the large, fluid-filled antrum, results in the expulsion of the oocyte surrounded by granulosa cells into the peritoneal cavity. This release is ovulation.
In the next section, you will follow the ovulated oocyte as it travels toward the uterus, but there is one more important event that occurs in the ovarian cycle. The surge of LH also stimulates a change in the granulosa and theca cells that remain in the follicle after the oocyte has been ovulated. This change is called luteinization (recall that the full name of LH is luteinizing hormone), and it transforms the collapsed follicle into a new endocrine structure called the corpus luteum, a term meaning “yellowish body” (see Figure 27.3.2). Instead of estrogen, the luteinized granulosa and theca cells of the corpus luteum begin to produce large amounts of the sex steroid hormone progesterone, a hormone that is critical for the establishment and maintenance of pregnancy. Progesterone triggers negative feedback at the hypothalamus and pituitary, which keeps GnRH, LH, and FSH secretions low, so no new dominant follicles develop at this time.
The post-ovulatory phase of progesterone secretion is known as the luteal phase of the ovarian cycle. If pregnancy does not occur within 10 to 12 days, the corpus luteum will stop secreting progesterone and degrade into the corpus albicans, a nonfunctional “whitish body” that will disintegrate in the ovary over a period of several months. During this time of reduced progesterone secretion, FSH and LH are once again stimulated, and the follicular phase begins again with a new cohort of early tertiary follicles beginning to grow and secrete estrogen.
The Menstrual Cycle
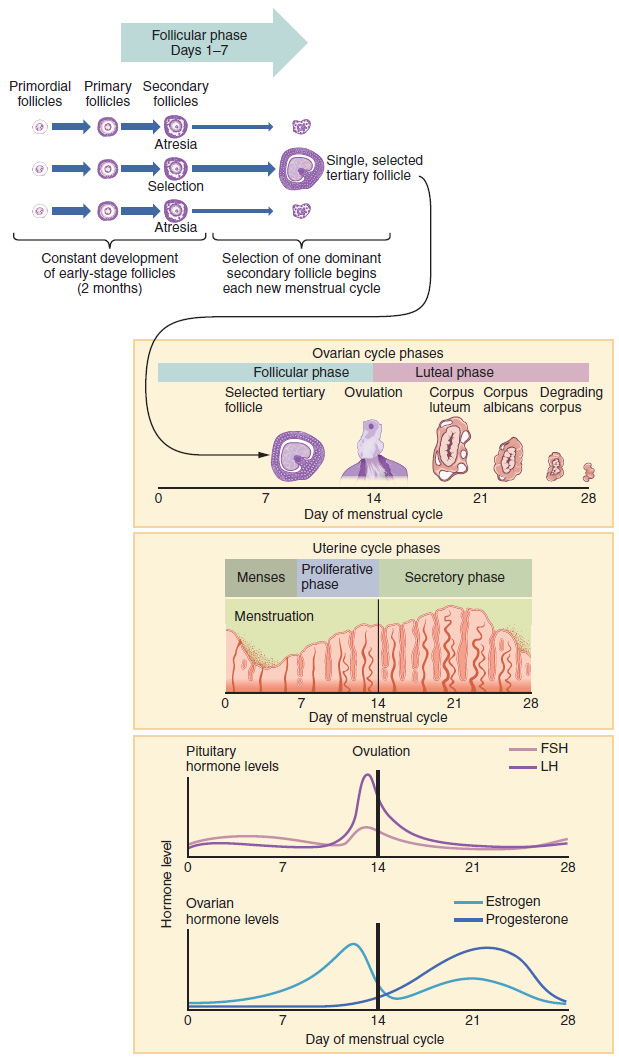
Now that we have discussed the maturation of the cohort of tertiary follicles in the ovary, the build-up and then shedding of the endometrial lining in the uterus, and the function of the uterine tubes and vagina, we can put everything together to talk about the three phases of the menstrual cycle—the series of changes in which the uterine lining is shed, rebuilds, and prepares for implantation.
The timing of the menstrual cycle starts with the first day of menses, referred to as day one of a woman’s period. Cycle length is determined by counting the days between the onset of bleeding in two subsequent cycles. Because the average length of a woman’s menstrual cycle is 28 days, this is the time period used to identify the timing of events in the cycle. However, the length of the menstrual cycle varies among women, and even in the same woman from one cycle to the next, typically from 21 to 32 days.
Just as the hormones produced by the granulosa and theca cells of the ovary “drive” the follicular and luteal phases of the ovarian cycle, they also control the three distinct phases of the menstrual cycle. These are the menses phase, the proliferative phase, and the secretory phase.
Menses Phase
The menses phase of the menstrual cycle is the phase during which the lining is shed; that is, the days that the woman menstruates. Although it averages approximately five days, the menses phase can last from 2 to 7 days, or longer. As shown in Figure 27.3.4, the menses phase occurs during the early days of the follicular phase of the ovarian cycle, when progesterone, FSH, and LH levels are low. Recall that progesterone concentrations decline as a result of the degradation of the corpus luteum, marking the end of the luteal phase. This decline in progesterone triggers the shedding of the stratum functionalis of the endometrium.
Proliferative Phase
Once menstrual flow ceases, the endometrium begins to proliferate again, marking the beginning of the proliferative phase of the menstrual cycle (see Figure 27.3.4). It occurs when the granulosa and theca cells of the tertiary follicles begin to produce increased amounts of estrogen. These rising estrogen concentrations stimulate the endometrial lining to rebuild.
Recall that the high estrogen concentrations will eventually lead to a decrease in FSH as a result of negative feedback, resulting in atresia of all but one of the developing tertiary follicles. The switch to positive feedback—which occurs with the elevated estrogen production from the dominant follicle—then stimulates the LH surge that will trigger ovulation. In a typical 28-day menstrual cycle, ovulation occurs on day 14. Ovulation marks the end of the proliferative phase as well as the end of the follicular phase.
Secretory Phase
In addition to prompting the LH surge, high estrogen levels increase the uterine tube contractions that facilitate the pick-up and transfer of the ovulated oocyte. High estrogen levels also slightly decrease the acidity of the vagina, making it more hospitable to sperm. In the ovary, the luteinization of the granulosa cells of the collapsed follicle forms the progesterone-producing corpus luteum, marking the beginning of the luteal phase of the ovarian cycle. In the uterus, progesterone from the corpus luteum begins the secretory phase of the menstrual cycle, in which the endometrial lining prepares for implantation (see Figure 27.3.4). Over the next 10 to 12 days, the endometrial glands secrete a fluid rich in glycogen. If fertilization has occurred, this fluid will nourish the ball of cells now developing from the zygote. At the same time, the spiral arteries develop to provide blood to the thickened stratum functionalis.
If no pregnancy occurs within approximately 10 to 12 days, the corpus luteum will degrade into the corpus albicans. Levels of both estrogen and progesterone will fall, and the endometrium will grow thinner. Prostaglandins will be secreted that cause constriction of the spiral arteries, reducing oxygen supply. The endometrial tissue will die, resulting in menses—or the first day of the next cycle.
Disorders of the Female Reproductive System
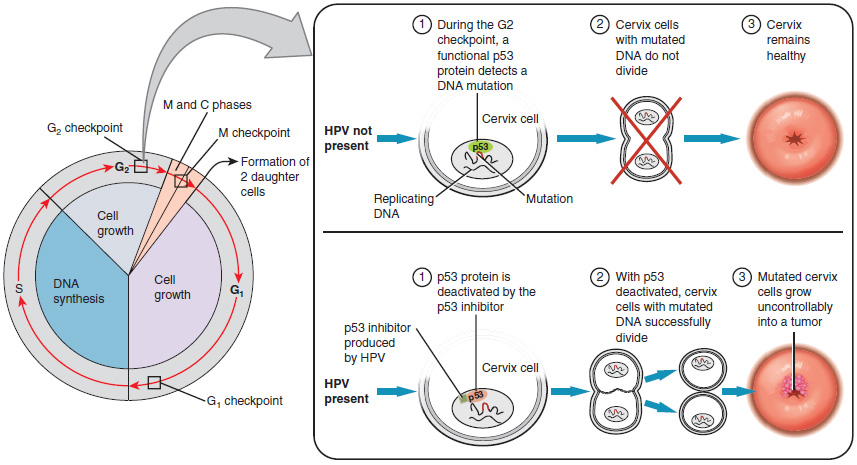
Research over many years has confirmed that cervical cancer is most often caused by a sexually transmitted infection with human papillomavirus (HPV). There are over 100 related viruses in the HPV family, and the characteristics of each strain determine the outcome of the infection. In all cases, the virus enters body cells and uses its own genetic material to take over the host cell’s metabolic machinery and produce more virus particles.
HPV infections are common in both men and women. Indeed, a recent study determined that 42.5 percent of females had HPV at the time of testing. These women ranged in age from 14 to 59 years and differed in race, ethnicity, and number of sexual partners. Of note, the prevalence of HPV infection was 53.8 percent among women aged 20 to 24 years, the age group with the highest infection rate.
HPV strains are classified as high or low risk according to their potential to cause cancer. Though most HPV infections do not cause disease, the disruption of normal cellular functions in the low-risk forms of HPV can cause the male or female human host to develop genital warts. Often, the body is able to clear an HPV infection by normal immune responses within 2 years. However, the more serious, high-risk infection by certain types of HPV can result in cancer of the cervix (Figure 27.3.5). Infection with either of the cancer-causing variants HPV 16 or HPV 18 has been linked to more than 70 percent of all cervical cancer diagnoses. Although even these high-risk HPV strains can be cleared from the body over time, infections persist in some individuals. If this happens, the HPV infection can influence the cells of the cervix to develop precancerous changes.
Risk factors for cervical cancer include having unprotected sex; having multiple sexual partners; a first sexual experience at a younger age, when the cells of the cervix are not fully mature; failure to receive the HPV vaccine; a compromised immune system; and smoking. The risk of developing cervical cancer is doubled with cigarette smoking.
The prevalence of cervical cancer in the United States is very low because of regular screening exams called pap smears. Pap smears sample cells of the cervix, allowing the detection of abnormal cells. If pre-cancerous cells are detected, there are several highly effective techniques that are currently in use to remove them before they pose a danger. However, women in developing countries often do not have access to regular pap smears. As a result, these women account for as many as 80 percent of the cases of cervical cancer worldwide.
In 2006, the first vaccine against the high-risk types of HPV was approved. There are now two HPV vaccines available: Gardasil® and Cervarix®. Whereas these vaccines were initially only targeted for women, because HPV is sexually transmitted, both men and women require vaccination for this approach to achieve its maximum efficacy. A recent study suggests that the HPV vaccine has cut the rates of HPV infection by the four targeted strains at least in half. Unfortunately, the high cost of manufacturing the vaccine is currently limiting access to many women worldwide.
Hormonal Birth Control
Birth control pills take advantage of the negative feedback system that regulates the ovarian and menstrual cycles to stop ovulation and prevent pregnancy. Typically they work by providing a constant level of both estrogen and progesterone, which negatively feeds back onto the hypothalamus and pituitary, thus preventing the release of FSH and LH. Without FSH, the follicles do not mature, and without the LH surge, ovulation does not occur. Although the estrogen in birth control pills does stimulate some thickening of the endometrial wall, it is reduced compared with a normal cycle and is less likely to support implantation.
Some birth control pills contain 21 active pills containing hormones, and 7 inactive pills (placebos). The decline in hormones during the week that the woman takes the placebo pills triggers menses, although it is typically lighter than a normal menstrual flow because of the reduced endometrial thickening. Newer types of birth control pills have been developed that deliver low-dose estrogens and progesterone for the entire cycle (these are meant to be taken 365 days a year), and menses never occurs. While some women prefer to have the proof of a lack of pregnancy that a monthly period provides, menstruation every 28 days is not required for health reasons, and there are no reported adverse effects of not having a menstrual period in an otherwise healthy individual.
Because birth control pills function by providing constant estrogen and progesterone levels and disrupting negative feedback, skipping even just one or two pills at certain points of the cycle (or even being several hours late taking the pill) can lead to an increase in FSH and LH and result in ovulation. It is important, therefore, that the woman follow the directions on the birth control pill package to successfully prevent pregnancy.
This work, Anatomy & Physiology, is adapted from Anatomy & Physiology by OpenStax, licensed under CC BY. This edition, with revised content and artwork, is licensed under CC BY-SA except where otherwise noted.
Images, from Anatomy & Physiology by OpenStax, are licensed under CC BY except where otherwise noted.
Access the original for free at https://openstax.org/books/anatomy-and-physiology/pages/1-introduction.

