21 3.3 EAE Murine Model and the Microbiome
Experimental Autoimmune Encephalomyelitis (EAE)
Experimental Autoimmune Encephalomyelitis (EAE) mice are a well-established model of MS where the induction of myelin antigens causes the spontaneous development of RRMS (DiSano et al., 2019). Similar to MS, EAE is characterized by cellular infiltration of autoreactive T-cells into the central nervous system and the demyelination of neurons (Zhang et al., 2011). Here is a short video of EAE mice who develop hind-limb paralysis upon myelin induction.
In sharing a similar etiology to MS, researchers have used EAE mice to discover potential novel factors that may contribute to the onset and progression of MS. A recent field of research has emerged demonstrating the role of the gut microbiome in the progression of EAE. It was found that germ-free mice were protected from EAE compared to specific pathogen-free mice (Arndt et al., 2014). However, when germ-free mice were transplanted with microbiota, they developed of a higher incidence of EAE compared to controls (Chu et al., 2018). Interestingly, it has also been demonstrated that treatment with antibiotics can delay the development of EAE and regulate abnormal T-cell responses (Chu et al., 2018).
From these studies, it is evident that the microbiota plays a role in EAE development. However, demonstrating these findings in humans subjects has been difficult as the microbiome is highly heterogeneous and is dependent on both genetic and environmental factors. Despite this, there has been a substantial body of evidence demonstrating a link between the microbiome and the brain, which we will describe in the next section.
Role of the Microbiome in MS
The gut microbiota is an extremely fascinating system that has many functions ranging from regulating host metabolism to producing bioactive molecules. It is incredibly diverse, with only a few phyla covering more than 160 species in the gut (Rinninella et al., 2019). The primary phyla in the gut are Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, and Verrucomicrobia. Due to its diversity and vast production of metabolites, it is not surprising that these bioactive molecules may influence our brain function. For example, in Parkinson’s disease patients it was found that the gut microbiome promoted motor and GI dysfunction (Sampson et al., 2016). Similarly, gut microbiota alterations due to antibiotic treatment reduced plaque-gliosis in a mouse model for Alzheimer’s disease (Minter et al., 2016).
In addition to these findings, recent studies demonstrate a potential role of the microbiome in MS. In a study conducted by Berer et al. (2017), it was found that mice developed a higher incidence of EAE when transplanted with microbiota from MS patients compared to healthy controls. As well, these mice had a reduction in IL-10 production and Sutterella, which is known to support an immunoregulatory profile. From this study it is evident that dysbosis likely contributes to the progression or development of MS. This hypothesis is further supported by the finding that treating mice with Clostridium butyricum and norfloxacin (antibiotic) ameliorated EAE, increased Treg responses, and reduced Th17 production (Chen et al., 2019).
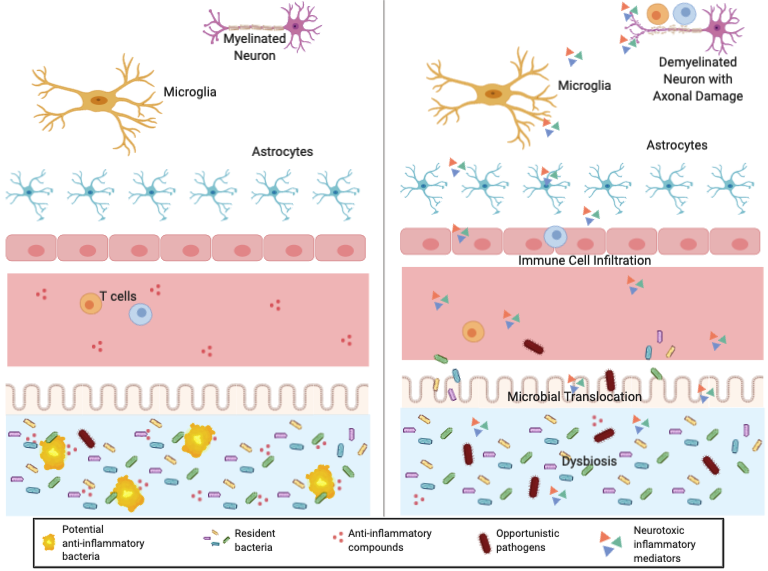
But how can bacteria in the gut cause inflammation in the brain? Gut bacteria actually plays a significant role in the CNS of animals by affecting blood brain barrier (BBB) permeability, microglial activation, limiting astrocyte pathogenicity, and expressing myelinating genes (Berer et al., 2017). This interaction between the gut and brain is called the gut-brain axis. The recent discovery of butyrate as a gut-derived metabolite that modulates circulating Tregs in mice demonstrates how the gut microbiota may affect Treg cell function and contribute to the development of autoimmune diseases. Figure 3 shows a healthy gut-brain axis (left) compared to the multiple sclerosis axis (right).
Healthy vs. Multiple Sclerosis
Present Pilot Study
Although there is a potential link between the microbiota and MS, as mentioned previously, studies are limited due to heterogentity. As such, there have not been many studies that aim to address the influence of the microbiota in human MS subjects. Despite these challenges, the authors of this study were motivated to determine the clinical and immunological effect of diet modulation on the gut microbiome in MS patients. They hypothesized that dietary components such as fiber, acting on the microbiota compostion, could, in principle, result in immune modulation and, thus, could be used to obtain beneficial outcomes for patients (Saresella et al., 2017). Neat right? Let’s see how the authors went about performing this experiment.
A microbrial imbalance of our natural gut flora that contributes to various diseases
The central nervous system (CNS) is one of the two major divisions of the nervous system which includes the brain and the spinal cord.
A filtering mechanism of the capillaries that carry blood to the brain and spinal cord tissue, blocking the passage of certain substances.
