42 4.2 Current Treatments and Limitations
Learning Objectives
By the end of this section, you should be able to:
- Have an overall understanding of current therapies for the treatment of left ventricular remodeling after myocardial infarction
- Differentiate between consolidated, promising and potential drugs for the treatment of MI
- Explain how potential drugs for the treatment of MI may work
- Describe the relationship between LV wall stress and wall thickness and pressure
- Describe La Place’s rule
- Describe how surgeries may prove to be effective in restoring the function of the heart
- Have an understanding of the limitations of the surgery as a treatment
- Explain the role of IGF-1 as a growth factor
- Explain cell-based therapies and how they might work
- Describe the existing limitations for cell-based therapies
- Name different biomaterial techniques currently used
With growing rates of myocardial infarction in Western countries, the leading cause of mortality, there is an increasing need for a suitable therapy that addresses the left ventricular remodeling. Some of the current treatments are small molecule drugs, surgery, cell-based therapies, and biomaterials. Even though some of these therapies show promising results in the lab, there are mixed outcomes when it comes to their practicality. This chapter will give an overview of some of these therapies and their expected outcomes.
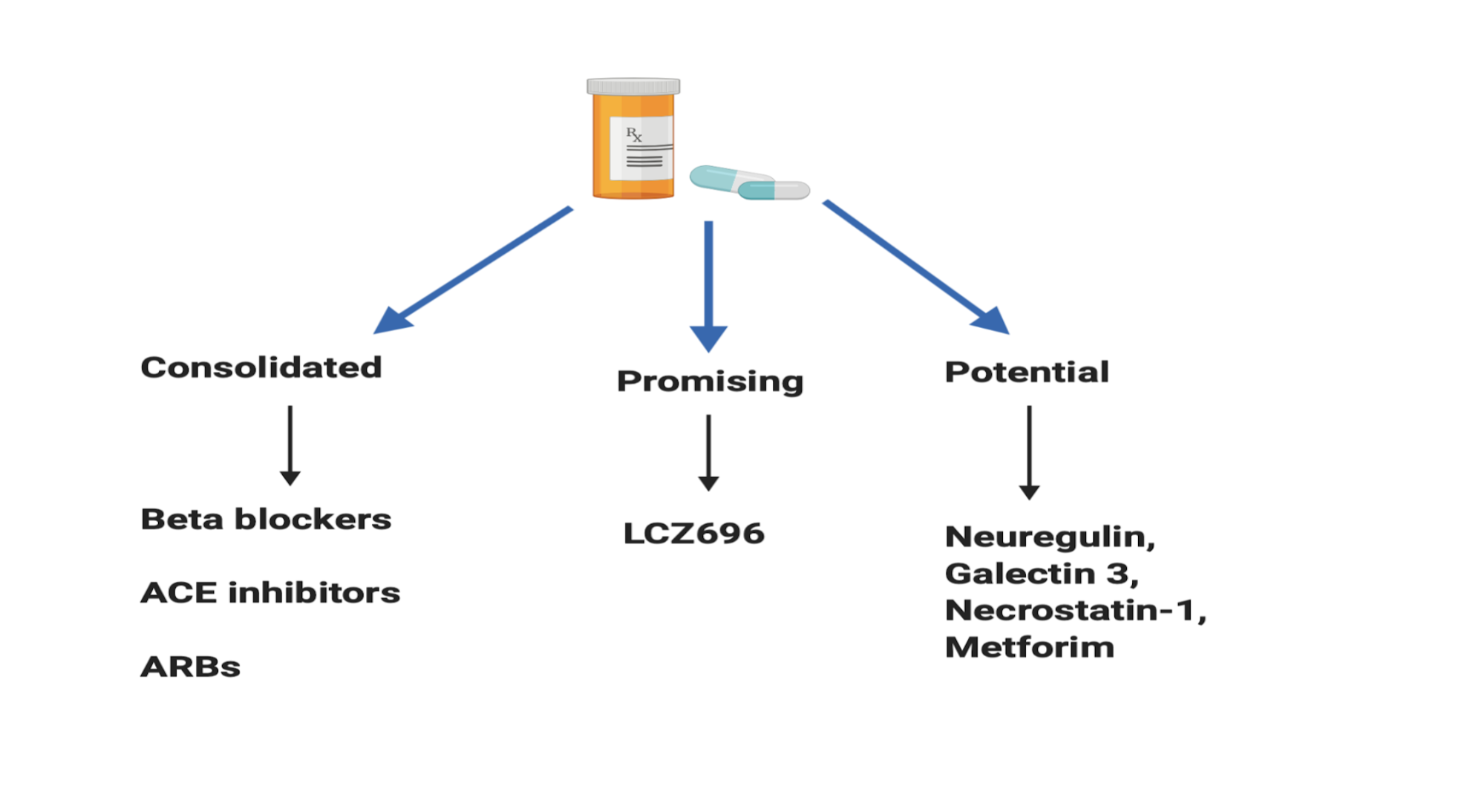
Current pharmacological drugs for the treatment of left ventricular remodeling (LV) can be divided into three categories: consolidated, promising and potential. Consolidated drugs include beta-blockers, angiotensin-converting enzyme inhibitors (ACE) and aldosterone antagonists. These drugs have successfully reduced cardiac remodeling in animal models (Sayer G, 2014., Albuquerque FN 2014., Florea VG 2014). They have also shown to be effective in clinical trials and are currently used as treatments for cardiac remodeling in patients with MI (Reis Filho JR, 2015). An example of a promising drug is LCZ696 which contains a valsartan molecule, an angiotensin receptor II antagonist, and sacubitril, an inhibitor of neprilysin. Neprilysin metabolizes natriuretic peptides such as adrenomedullin, urodilatin, and bradykinin (Azevedo et al., 2016). Von Leuder et al. show a reduction in fibrosis and ventricular cavity dilation in experimental studies using LCZ696 (2015). For potential drugs, fibrosis, cell apoptosis, and modulation of the inflammatory processes are attractive targets. For instance, necrostatin-1 prevents apoptosis of cells by inhibiting caspase-8, a cysteine protease involved in apoptotic signaling (Azevedo et al., 2016) and Galectin-3 inhibition leads to a decrease in collagen composition and thus reduces fibrosis in the infarct area (de Boer RA, 2014). Nevertheless, with drugs, there are also unwanted side effects that may negatively affect a patient’s quality of life.
Myocardial infarction leads to an adaptive response that manifests itself as an increase in Left Ventricular (LV) volume which serves to maintain the cardiac output as the ejection fraction decreases. In this early stage, cardiac remodeling and hypertrophy of cardiomyocytes leads to geometric distortion, heart chamber enlargement and increased wall mass (Pfeffer et al. 1990). Ventricular performance decline soon follows fibrosis, collagen synthesis and remodeling of the extracellular matrix in the region near the infarct area (Braunwald E et al., 1990). LV wall stress is inversely proportional to the wall thickness and directly proportional to pressure and internal radius of LV. According to La Place’s rule radius of a sphere is proportional to the cube root of volume while wall stress is proportional to pressure x radius and inversely proportional to wall thickness. An increase in wall thickness decreases the wall stress and an increase in radius leads to an increase in wall stress. Surgeries that are aimed at reducing scar tissue and reshaping the distorted chamber and therefore reducing wall stress can prove to be effective in increasing cardiac function (Castelvecchio S, 2019). For instance, surgical ventricular reconstruction (SVR) technique improves LV systolic function, reduces LV volume and results in a high survival rate of up to five years (Menicanti L et al., 2007). Nevertheless, despite the promising results, not every patient can benefit from this surgery owing to factors such as the requirement for a high level of surgical expertise, heart failure symptoms, LV geometrics and the functioning of the mitral valve. Not all patients meet the required parameters suitable for a successful surgery, thus showing the limit of the effectiveness of the surgical procedure for all patients.
External Website
Watch this video to learn about LaPlace’s law to understand the effect of radius, pressure and wall thickness on the wall stress in left ventricles. The video also discusses why the heart does not rip apart despite the pressure that it endures.

External Website
Previous studies show the promising role of growth factors in treating cardiac remodeling after MI. IGF-1 is an example of a growth factor that improves the survival and growth of cardiomyocytes. Overexpression of IGF-1 protects against cardiomyocyte apoptosis and dilation of the left ventricle (Li Q et al., 1997, Torella D et al. 2004). It is also involved in the maintenance of transported embryonic stem cells in an infarcted heart (Kofidis T et al. 2004). One property of IGF-1 is its ability to diffuse long distances in order to function as a hormone and affect nearby tissues; however, this property may hinder IGF-1’s retention(Fraidenraich D, et al. 2004). Davis ME et al. designed a biotin sandwich method that allows for the self-assembly of IGF-1 in the myocardium and thus its longer retention in the tissue. They combined streptavidin, which has a high affinity for biotin, and biotin-IGF-1. This tethered IGF-1 product was able to show a reduction of cardiomyocyte apoptosis, bioactivity in vivo, and cell therapy improvement after infarction.
Cell-based therapies look at regenerating the heart via transplantation of cells into the tissue. Such transplantations include skeletal myoblasts, multipotent adult stem cells, and engineered tissues. Cellular cardiomyoplasty is a term often used to describe these types of transplantations. There are several challenges associated with cell-based transplantation therapies including but not limited to extensive death of transplanted cells, cell delivery complications and barrier-induced nature of scar tissue. Previous studies show that up to 90% of cells delivered to the area die within the first week (Mangi AA et al., 2003, Neff T et al., 2002). Around 90% of delivered cells are lost due to the leak from the injection site and current literature show variable cell retention. As a result, variable retention makes the graft size unpredictable (Muller-Ehmsen et al., 2002). Research also shows that the scar tissue acts as a barrier to graft integration (Reinecke et al., 1999) and prevents the formation of electrochemical junction between transplanted cardiomyocytes and the host myocardium. These junctions are required for the proper and synchronous beating of the heart (Laflamme MA et al., 2005). These disadvantages make cell-based therapies an unpredictable treatment for the damaged heart.
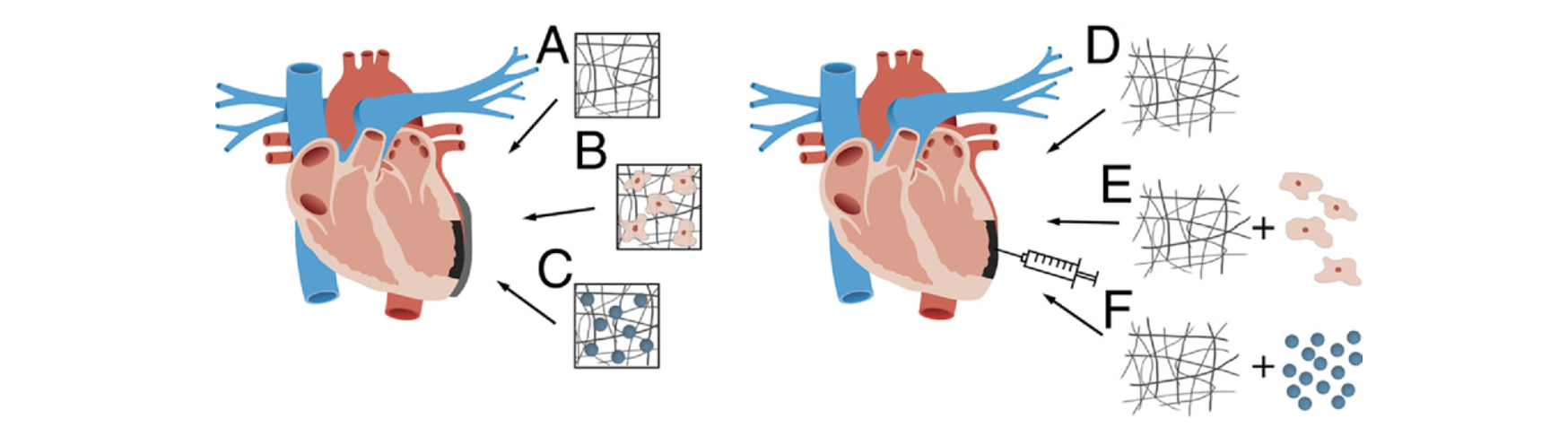
Biomaterial techniques currently used are injectable approaches, LV restrains and cardiac patches. One way to counter the adverse remodeling of LV after MI is to restrain the wall via biomaterial support. Restraining the wall preserves cardiac function, cardiac geometry and increases collagen ( Kelley et al., 1999; Bowen et al., 2001). A notable disadvantage of LV strains is the invasive nature of the procedure as it requires surgery. Thus researchers have lost interest in this procedure as it is evident by the low number of research in this area (Rane AA, et al. 2011). Injectable biomaterials are a less invasive approach that shows improvement in neovascularization, cardiac function and a reduction in infarct tissue size (Rane AA, et al. 2011). In their experiment, Christman et al. injected fibrin glue in the infarct area which resulted in restored geometry and thus improved function of the LV (2003). Cardiac patches show promising results in animal models by increasing the differentiation of cells and cardiac function (Sarig U et al., 2011). Nevertheless, challenges such as making patches of the right thickness still remain unsolved.
Key Takeaways
- surgical procedures are not available to everyone
- evidence for the effectiveness of cell-based therapies remain scant
- Biomaterials are the most promising of all the treatments

