66 5.12: Critical and Comparative Analyses
Critical Analysis
This study offered insight into the cellular mechanisms initiated by e-cigarette exposure (Zahedi et al.). Importantly, the authors investigated the effects of both e-liquids and aerosols from electronic cigarettes (Zahedi et al). This is significant because the applicability of results from in vitro studies using e-liquids has been questioned, due to the potential change in chemical composition once the e-liquid evaporates into a vapour (Farsalinos et al.). Also, the authors were careful to use nicotine concentrations that were physiologically relevant to what is experienced in vivo (Rose et al.; Zahedi et al.). Another strength of this study was the author’s thorough approach. For example, to confirm the involvement of nicotinic receptors and calcium influx, the authors used inhibition, such as nAChR blockers and calcium chelators, to see if their observations differed according to their expectations (Zahedi et al.).
As science is a critical practise, it is necessary to recognize the weaknesses of this study. A glaringly evident concern is whether or not their methodology reflects in vivo exposure experienced by e-cigarette users (Farsalinos et al.) Evidently, this study was strictly in vitro, which limits the translatability to what occurs in an interconnected, living system. This prevented the accurate recapitulation of chronic use of e-cigarettes (Zahedi et al.). Another unrealistic aspect is the production of aerosols from only one puffing topography, a variable that may vary greatly between e-cigarette users (Behar et al.; Zahedi et al.).
Also, the authors relied mainly on morphology to detect SIMH. As the genes involved in mitochondrial fusion (and its counterpart process, mitochondrial fission) are known, the authors could have evaluated regulation of these genes to further validate their findings (Ballweg et al.).
Comparative Analysis
The results of this study are supported by previous research, with one exception: the role of nicotine. As the authors attributed the observed effects to nicotine, similar results would be expected from research examining the cellular effects of another popular nicotine delivery product: traditional cigarettes (Zahedi et al.). Indeed, when alveolar epithelial cells were treated with cigarette smoke extract, their mitochondria displayed SIMH, as assessed by both morphological and genetic markers (Ballweg et al.). Similarly, SIMH was observed as a result of thirdhand cigarette smoke exposure in murine neural stem cells (Bahl et al., 2016). Combined with the observation that nicotine alone induces SIMH (Zahedi et al.), it is rational to infer that mitochondria respond to nicotine by undergoing SIMH.
However, the opposite process of SIMH, mitochondrial fission–in which mitochondria are fragmented–has occurred in embryonic carcinoma cells exposed to nicotine (10 μM), which was associated with a downregulation of mitochondrial fusion genes (Hirata et al.). Perhaps this contrasting response to nicotine may be explained by different pathways invoked in normal versus cancerous cell lines. Duration of exposure and concentration may other variables involved in mitochondrial response to nicotine: when hippocampal neurons were treated with 10 μM of nicotine, fusion was observed to increase with time (Godoy et al.). Yet, treatment with 25μM of nicotine did not significantly increase mitochondrial fusion, even with longer exposure duration (Godoy et al.). Evidently, the cellular relationship between nicotine and SIMH isn’t as definite as it may seem (Zahedi et al.).
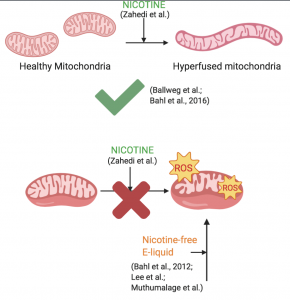
Furthermore, not all studies corroborate the conclusion that nicotine is independently responsible for the oxidative stress caused by e-liquids. When tested on human embryonic stem cells and murine neural stem cells, many e-liquids were found to by cytotoxic, even if their nicotine concentration was 0 mg/ml (Bahl et al., 2012). Additionally, nicotine content was irrelevant to cytotoxicity of e-liquids and serum from e-cigarette users on human-induced pluripotent stem cell-derived endothelial cells (Lee et al.). Treatment of human monocytes with nicotine-free e-cigarette flavourings and e-liquids resulted in reduced cell viability and elevated ROS production (Muthumalage et al.). Despite the well-established fact that nicotine is harmful to cells, the toxicity of e-liquids cannot be solely attributed to nicotine, as these substances have been demonstrated to be cytotoxic without nicotine (Bahl et al., 2012; Lee et al., Muthumalage et al.).
It is possible that while nicotine is a cellular stressor that can cause SIMH, other components in e-liquids or vape aerosols are independently deleterious. To test this, Zahedi and collaborators should have included a treatment group exposed to e-liquids with a nicotine concentration of 0 mg/ml. If no adverse effects were observed in the cells (which seems highly unlikely based on other research (Bahl et al., 2012; Lee et al., Muthumalage et al.), then they could have more definitively implicated nicotine as the sole cause in vaping-induced pathology.
