7.1.1 – Cell and Molecular Hallmarks of Alzheimer’s Disease
Alzheimer’s Disease (AD) is caused by damaged brain cells which affect the optimal function of the brain. Since the brain is the centre of intelligence, memory and senses, damage to the brain cells causes very significant symptoms such as loss of memory, a decline in thinking abilities, difficulties in completing familiar tasks as well as unpredictable behaviour.
In the early discovery of Alzheimer’s Disease, Dr. Alzheimer, a German neurologist, found multiple abnormal clumps (amyloid plaques) and tangled bundles of fibres (neurofibrillary tangles) in the brain tissue of a deceased woman who died from an unusual mental illness. Further research showed that the plaques can block the impulse transfer between neurons and hence causes loss of connections when they accumulate between the dendrites and synapses of neurons. This loss of connection of neurons will then affect memory storing, verbal, thought and muscle responses of individuals. As it progresses, further neuronal loss occurs which may cause individuals to be bedridden. This happens because the muscles are unable to move as there is a lack of signal transmission.
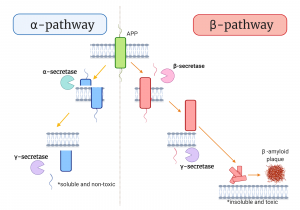
The clumps that were initially observed by Dr. Alzheimer are known as β-amyloid plaques. It is one of the most researched bio-markers in the study of AD. These plaques are produced from the cleaving of the amyloid precursor protein (APP) which is a glycoprotein expanding across the membrane layer of the neurons. APP is very important to the human body as it was reported to have functions in cell repair after injury. In order to participate in cell repair, APP needs to be cleaved. In healthy individuals, the APP will undergo the α-pathways, in which it will be cleaved by the α-secretase enzyme to produce soluble N-terminal sAPPα. The remaining 10kD C-terminal will further be cleaved by the γ-secretase to produce 3 kD p3 fragment, which is non-toxic and hence allow the cell to undergo repair.
However, in individuals with AD, the cleaving of the APP is done through the β-pathways. APP cleaved by β-secretase will produce two fragments which are the sAPPβ and an 11.5 kD fragment. The 11.5kD fragment will further be cleaved by γ-secretase to produce Aβ peptides. The Aβ peptides are normally composed of about 40 to 42 amino acids. Aβ40-42 peptides are insoluble fragments hence can accumulate when present in abundance in the human body. When these peptides aggregate outside of neurons, they are known as the amyloid β-plaques. An in-vitro study reported that these plaques were associated with causing fibrillogenic and toxic properties to the neuron cells grown in the experiment.
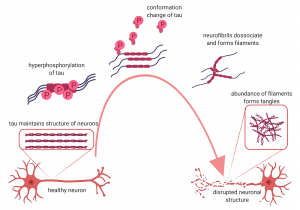
Similar to the plaques, the presence of neurofibrillary tangles has also become the center of studies around AD. Nevertheless, the plaques usually aggregate outside of the neurons while the neurofibrillary tangles occurred within the axon of the neuron itself. The tangles happened due to the hyperphosphorylation of tau proteins. In healthy individuals, the tau proteins are important to stabilize the microtubule making up the axon of the neuron. Tau can be phosphorylated to provide room for flexibility in the neuronal growth. However, tau protein is also susceptible to hyperphosphorylation which may cause a change in the conformation of tau protein. This will then cause neurofibrils to dissociate from the microtubules, form filaments and precipitate within the neurons.
Recent studies had classified other new hallmarks in AD. One of them includes the mitochondrial and oxidative damage to the brain cells due to increased markers of oxidation. When oxidation increases, this will lead to competitive inhibition of the antioxidant enzymes, hence causing a decrease in the enzymatic activity that protects neurons from being damaged. Studies also have reported that amyloid deposits may also be affiliated with the activation of microglia and astrocytes. The activation of these two proteins will, in turn, activate the inflammatory pathways and induce cytokines release, causing further neurodegeneration of cells in the brain.
Lastly, the Apolipoprotein E (apoE) was also classified as the hallmarks of AD. This protein is associated with the apoE ɛ4 allele. In AD patients, the apoE ɛ4 gene was observed to be over-represented while the apoE ɛ2 allele was reported to be under-represented. Pathologically, the excess of apoE ɛ4 was also implicated in the early onset of AD to causing an increase in the amyloid-β deposition. Even though the specific function of apoE is still unclear, the normal regulation of this protein has been associated with brain development, nerve growth, immune response, cholesterol distribution after inflammation, as well as in providing anti-oxidative protection to neuronal cells.
