52 6.5 Critical Analysis: Limitations of Statin Drug Trials
Questions You Will Be Able to Answer After Reading This Section:
- What factor can influence Glomerular Filtration Rate (GFR)?
- What is a possible benefit/limitation of using a run-in period in a clinical trial?
Kimura et al. (2017) Study Limitations:
1. Diet Therapy Not Clarified
Throughout the 2-year treatment, all participants were allocated identical diet therapies (Kimura, et al, 2017); however, the diet was never disclosed and caloric intake was never recorded; this poses a limitation in terms of accuracy. Specifically, it remains unclear the extent to which diet affected the eGFR of both groups.
While it is well known that diet and lifestyle have an influential role on cholesterol levels, the relationship between these two parameters and CKD is not fully understood. However, recent literature has shown a possible correlation between diet intake and CKD risk. For instance, Juraschek et al. (2016) found that non-CKD patients fed a low carb, high protein diet had increased Glomerular Filtration Rates (GFR), and thus may be at an increased risk of improper kidney functioning. Furthermore, a recent longitudinal analysis also showed a correlation between increased CKD risk and a low carbohydrate, high protein diet (i.e. a diet consisting of saturated fat and protein from animal products) (Farhadnejad, Asghari, Emamat, Mirmiran, & Azizi, 2019). Therefore, because the diet was never clarified, it remains unclear how the diet affected the eGFRs.
Diet not only has an influential role on GFR, but it also can influence the efficiency of atorvastatin. For instance, the effectiveness of atorvastatin is increased when paired with a low-cholesterol diet consisting of omega-3 fatty acids (Rosenthal, 2000). Again, because the diet was never disclosed, the food consumed may have influenced the efficiency of atorvastatin.
2. No Isolation of Atorvastatin
Throughout the 2 year treatment, all participants were allowed to take other lipid-lowering drugs if the target LDL-C level of 100 mg/dl was not reached (Kimura, G. et al, 2017). While a variety of drugs could be taken, 70% of all participants were taking RAAS inhibitors and 79% of participants in Group C were taking ezetimibe (Kimura, G. et al, 2017). The number of participants that solely took atorvastatin was never disclosed and all participants were taking different lipid-lowering drugs and at different doses throughout the study. Therefore, it cannot be concluded that atorvastatin was the sole drug that reduced serum LDL or TG levels in Group A, since participants were taking alternative lipid-lowering drugs whilst taking atorvastatin.
Current Limitation in Statin Drug Trials
Run-In Periods
In randomized statin clinical trials, run-in periods may occasionally be used to exclude participants after the treatment is given and prior to the randomization taking place.
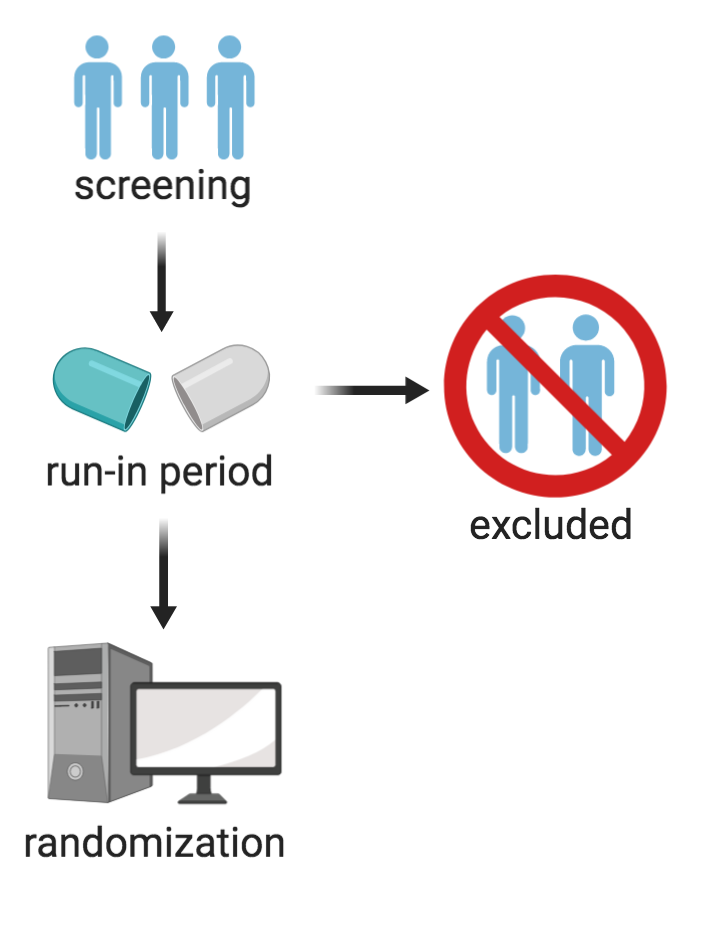
For instance, if a participant reacts well to a placebo, or if they do not react well to a particular drug, they may be excluded without the researchers having to record the reasons as to why (since the exclusion takes place prior to randomization) (Pablos-Méndez, Barr, & Shea, 1998). A run-in period is primarily used to reduce the number of exclusions, allowing for a greater study population to be incorporated in the final results (Laursen, Paludan-Müller, & Hróbjartsson, 2019). However, one of the limitations associated with this trial method is validity. If participants experience side effects to a particular drug during the run-in period, they can be excluded from the trial. Because these participants are excluded, the study population, in terms of side-effects, no longer reflects the real-world patients for that drug (Pablos-Méndez et al., 1998). Furthermore, the benefits of the drug become exaggerated and the side effects are reduced.
Run-in periods are disproportionately found in statin drug trials conducted or funded by large pharmaceutical industries. For instance, Laursen et al. (2019) looked at 25 trials that included a run-in period and found that 16 of the 25 were industry trials. It was also found that 92% of these trials had insufficient records for the run-in periods – 72% of the trials did not state reasons for participant exclusion, and patient characteristics were also excluded (Laursen et al., 2019). Run-in periods are relatively common in randomized statin drug trials and thus, may fall victim to the limitations of validity. A study conducted by Fralick et al. (2018) aimed to assess whether the discrepancy between statin drug trials and real-world patients, in terms of side effects, could be due to run-in periods and found that half of the trials analyzed did not include reasons for exclusion for the run-in period; however, when it was recorded, the reason was poor adherence (Fralick et al., 2018).
Test Your Knowledge!
