31 2.4: Synaptic Dysfunction in Alzheimer’s Disease
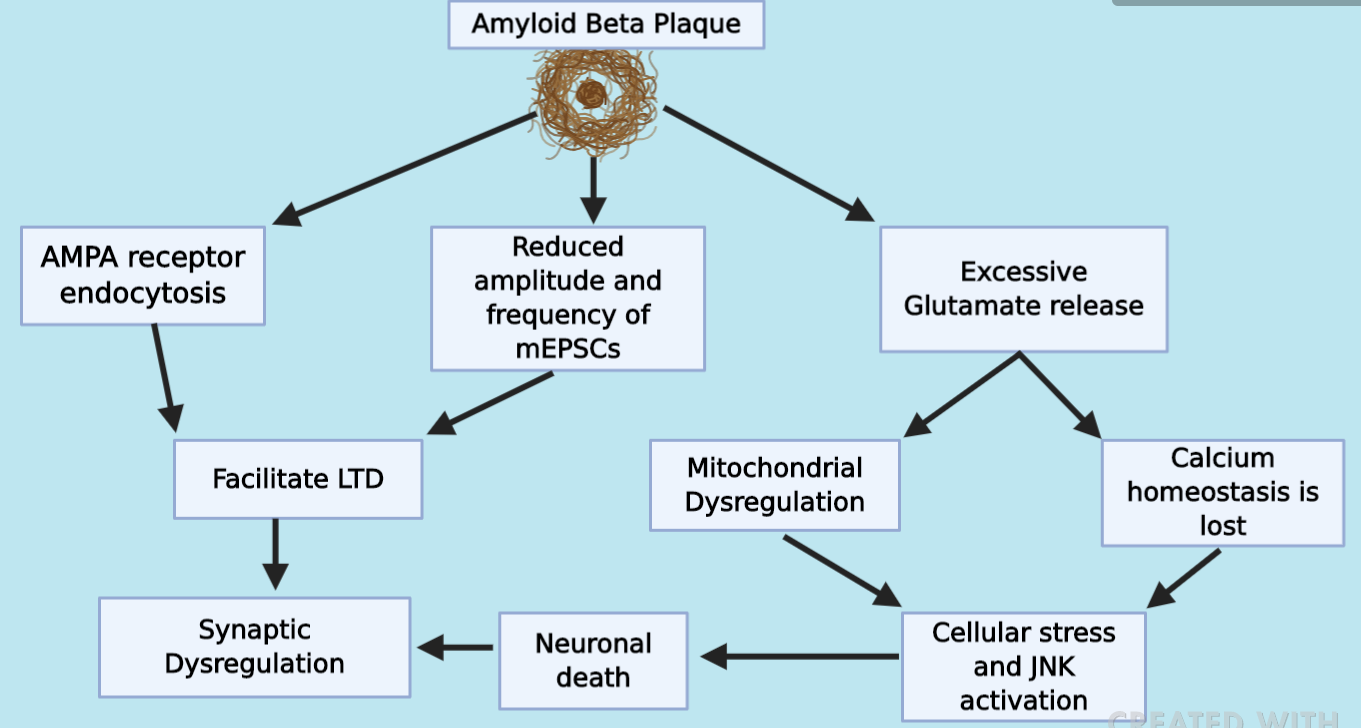
Synaptic dysfunction is a disturbance of intrinsic and/or biological processes involved in a synapse. Specifically in Alzheimer’s Disease, amyloid-β plaques play a multifactorial role in synaptic dysfunction by contributing to LTD formation, exocytoxicity leading to cellular stress and neuronal death. While the direct mechanisms are not known, amyloid-β plaques are linked to endocytosis of NMDA receptors on the postsynaptic membrane and a reduction in dendritic spine density leading to a diminished synaptic transmission and facilitating LTD (Shankar et. al, Marsh et. al). Amyloid-β was also reported to reduce the frequency and amplitude of mEPSCs fired from the presynaptic neuron, negatively impacting the strength of the synaptic efficiency as the postsynaptic neuron is less primed to fire, thus promoting LTD (Yin et. al).
Additionally, amyloid-β plaques affect the presynaptic neuron by increasing glutamate vesicle release, which would facilitate LTP but only temporarily. As glutamate is released in excessive amounts, neuronal mitochondria begin to dysfunction and cellular stress begins in the presynaptic neuron. Furthermore, the increased vesicle release generates free radicals and disrupts calcium ion homeostasis leading to cellular stress in the postsynaptic neuron. Cellular stress signals lead to the activation of the stress kinase JNK, triggering apoptosis thus promoting LTD as neuronal death leads to reduced synaptic transmission and overall synaptic dysfunction (Marsh et al).