33 2.6: Endophilin A
Endophilin A consists of 3 homologues that are cytosolic proteins and are expressed highly in the nervous system. But, endophilin 1 only being expressed in the brain, particularly at cell bodies and synaptic sites, localized at the presynaptic terminal, endophilin 2 in many tissues, and 3 in the brain and testes.
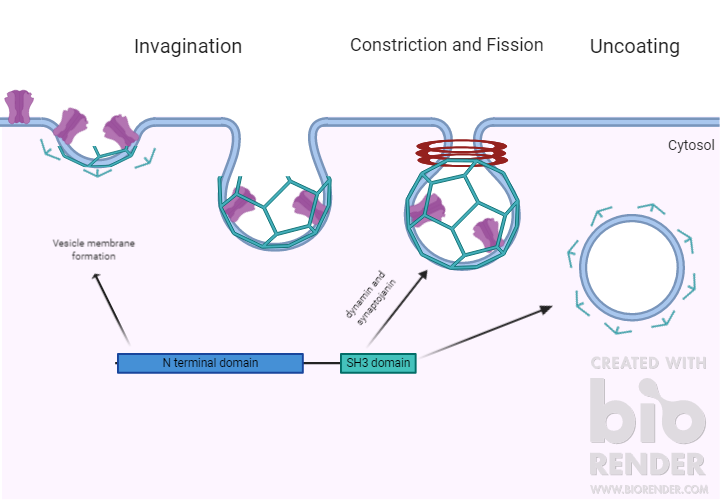
Endophilin A has an important role in the transport of synaptic vesicles. It is involved in the endocytosis pathway of clathrin-coated vesicles at synaptic sites. It contains a C terminal Src homology 3 (SH3) domain, which binds to proline-rich domains in other proteins that are involved in endocytosis, like dynamin and synaptojanin. As shown by a study from Gad et al., a disruption of the SH3 domain’s interaction with the proline-rich domain of other endocytic proteins impairs vesicle endocytosis at the synapse. From their experiments, they found endophilin was critical in the process of endocytosis, for fission, in the formation of the clathrin-coated vesicle, and for also uncoating of the vesicles. Endophilin’s N-terminus is also required for endocytosis. The N-terminus is involved in the formation of vesicles through interactions with lipids in the membrane that affect curvature.
In addition, as Verstreken et al. shown by mutants of endophilin, endophilin is required presynaptically for clathrin-dependent endocytosis. They found that the number of synaptic vesicles and miniature excitatory potentials is drastically decreased in endophilin mutants. In these mutants, they proposed that the kiss and run mechanism was able to maintain neurotransmission in the absence of clathrin-mediated endocytosis for synaptic vesicle retrieval, so endophilin mutation does not block neurotransmitter release.
Key Takeaways
- Endophilin is crucial for synaptic endocytosis in the nervous system
- Endophilin acts on multiple steps of the clathrin-mediated endocytosis pathway
In more recent studies, endophilin 1 expression has been linked with Alzheimer’s disease. A study done by Ren et al. found that in addition to the overexpression of amyloid-β, it was identified that endophilin 1 protein levels were also increased in Alzheimer’s patients. They also found the due to the increase in endophilin 1 expression, JNK stress kinase is activated. The activation of JNK decreases neuron viability, subsequently leads to cell death from the activation of the apoptotic pathway. A recent study from Yin et al. also shows that synaptic dysfunction occurs as endophilin 1 is highly expressed. However, a detailed mechanism of the pathway in which endophilin-1 leads to JNK activation requires further research.
