16 1.3 Future Directions
Future Directions: What’s Next?
Testing Other Cancer Cell Lines
The next steps in demonstrating the possible anti-NSCLC tumour properties of vitexin include testing vitexin on mice that have induced tumour growth resembling other types of lung cancer and see if the results are similar. This includes using cell lines that resemble squamous cell carcinoma or large cell carcinoma and seeing if vitexin has an effect of different types of lung cancer. A study done by Zhang et al. (2016), reported a transplantable squamous cell carcinoma cell line named ‘SK-MES-1’ that could be used for these tests.[1] These researchers studied the potential therapeutic effects of lobaplatin on SK-MES-1 lung squamous cancer carcinoma (LSQCC) cells in vitro and in vivo. Lobaplatin is a platinum compound that has shown anticancer properties in other tumours. [2] Since LSQCC is the second largest subtype of NSCLC, it is of interest to see if vitexin can potentially induce anti-tumour activity on this type of cancer.
Other NSCLC Cell Lines
Several different cell lines have been used to study NSCLC in vitro, some of these include:
- Adenocarcinomas – A549, H1650, and SPC-A1
- Large cell undifferentiated carcinomas – H460
- Squamous cell carcinomas – 95C, 95D, H226, H520, and SK-MES-1
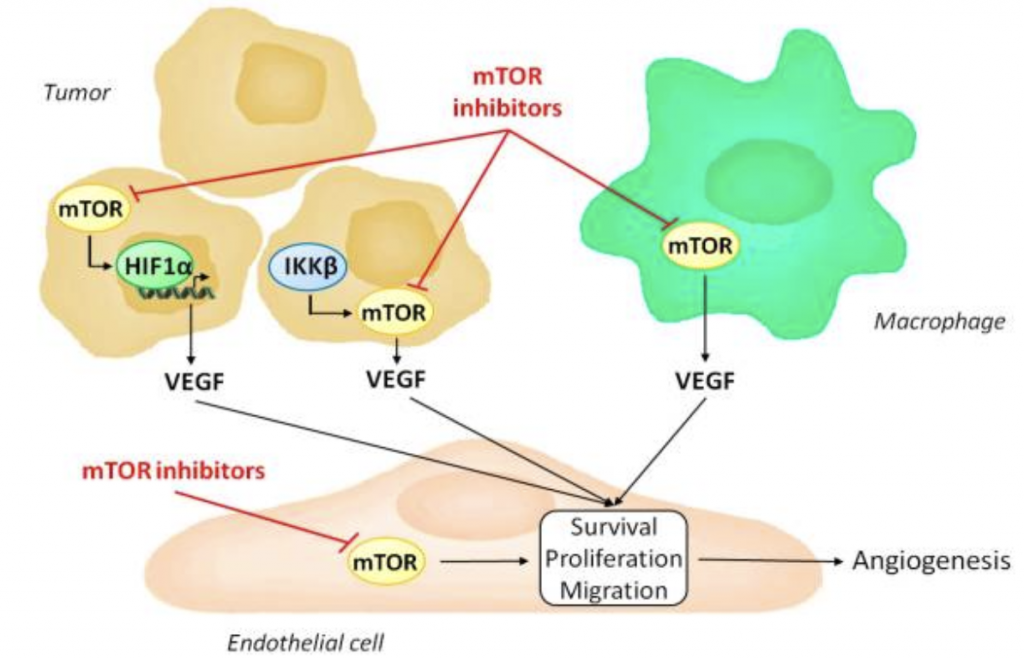
Downstream Molecules of PI3K/Akt/mTOR pathway
Vitexin should be tested to see if it can reduce the downstream molecules of the PI3K/Akt/mTOR signalling pathways to confirm its effects. For example, the mTOR pathway is involved with downstream molecules that are involved in angiogenesis, such as hypoxia induced factor 1 alpha (HIF1α) and vascular endothelial growth factor (VEGF). These downstream molecules of the pathway can be tested for to confirm that vitexin is actually inhibiting this pathway. If vitexin inhibits this pathway, these molecules should be downregulated as well. Research on this can be done through an angiogenesis assay.
Inhibition of the Angiogenesis Pathway by mTOR Inhibitors
Explore the Possibility of Tumour Resistance to Cancer Therapy
A review study done by Pistritto et al. (2016) discussed tumour resistance to therapies that stems from the deregulation of the regulators of apoptosis and because the study by Liu et al. (2019) is quite short in duration, the results may be different if the study was longer because of the possibility of cancer cell resistance to therapies.[5] Tumour cells can acquire resistance to apoptosis by the expression of anti-apoptotic proteins such as Bcl-2 or by the down-regulation or mutation of pro-apoptotic proteins such as Bax.[6] This could be tested through a longer study with A549 cells because a mouse model would likely die from the tumours over time. Tests for resistance could be conducted to see if vitexin results in resistance to apoptosis.
As for the future of cancer cell resistance to treatment, researchers in Northwestern University have discovered a way to develop anti-resistance for cancer cells in their lab.
Fighting Cancer’s Resistance to Treatment
- Zhang H, Chen R, Yang S, Liu W, Li K, Zhang H, Zhu X, Chen B. Lobaplatin for the treatment of SK-MES-1 lung squamous cell line in vitro and in vivo. OncoTargets and Therapy. 2016;Volume 9:4215–4224. ↵
- Zhang H, Chen R, Yang S, Liu W, Li K, Zhang H, Zhu X, Chen B. Lobaplatin for the treatment of SK-MES-1 lung squamous cell line in vitro and in vivo. OncoTargets and Therapy. 2016;Volume 9:4215–4224. ↵
- Zhu, J. et al. CD73/NT5E is a target of miR-30a-5p and plays an important role in the pathogenesis of non-small cell lung cancer. Mol. Cancer 16, 34 (2017). ↵
- Faes S, Santoro T, Demartines N, Dormond O. Evolving Significance and Future Relevance of Anti-Angiogenic Activity of mTOR Inhibitors in Cancer Therapy. Cancers (Basel). 2017 Nov 1;9(11):152. ↵
- Pistritto G, Trisciuoglio D, Ceci C, Garufi A, Dorazi G. Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging. 2016;8(4):603–619. ↵
- Pistritto G, Trisciuoglio D, Ceci C, Garufi A, Dorazi G. Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging. 2016;8(4):603–619. ↵
The formation of new blood vessels. In cancer, these new blood vessels provide a way for cancer cells to travel to other parts of the body.
