10 1.2 Western Blot and the mTOR Pathway
Brief Overview
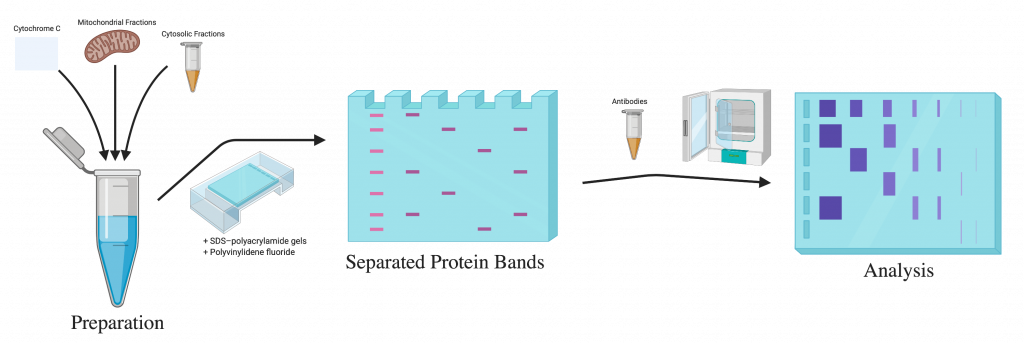
Western blot is a widely-used technique that identify specific proteins in a sample. The whole process consists of 3 main steps:[1]
- Separating proteins by size
- Transferring the proteins to a membrane
- Marking the specific proteins on the membrane using antibodies
This laboratory technique can separate the specific protein by size, transfer to a solid support and mark the target proteins using antibodies. In a western blot, the proteins are separated based on their molecular weight by gel electrophoresis. They are then transferred to a membrane that produces a band for each protein. The membrane is incubated for a certain amount of time with the specific antibodies to detect the protein of interest. The antibodies that are not bound to a specific protein are washed off, leaving only the bound antibodies. Then, the antibodies are detected, and since they are only bound to the protein of interest, only one band on the blot should be visible.
Experiment Protocol
The total protein was extracted using a radioimmunoprecipitation assay lysis buffer. For detection of cytochrome c, the mitochondrial and cytosolic fractions were prepared using the Mitochondria/Cytosol Fractionation kit. They grabbed equal amounts of protein from each sample that was separated by an SDS- polyacrylamide gel and electrotransferred to the polyvinylidene fluoride membranes. After being blocked in a solution consisting of 5% non-fat dried milk in PBS with Tween-20, the membranes were ready for the next set of treatments. The membranes were incubated at 4oC overnight with specific antibodies against Bcl-2, Bax, caspase-3, cytochrome c, p-PI3K, PI3K, Akt and GAPDH. After the incubation with primary antibodies, another incubation with HRP-conjugated secondary antibodies at room temperature for 1 hour followed. The proteins were detected using an enhanced chemiluminescence kit.
Sidebar: How to Analyze Western Blots
Experiment Overview
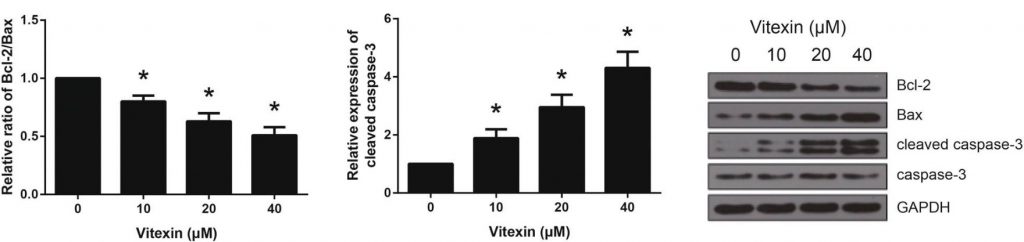
To further support the hypothesis that vitexin induces apoptosis in A549 cells, the researchers investigated the expression levels of proteins related to apoptosis using western blot analysis.
In this experiment, the researchers were looking to identify and quantify the expression of the proteins Bcl-2, Bax, and cleaved caspase-3. Bcl-2 and Bax are both members of the Bcl-2 family of proteins, which help regulate the apoptosis pathway. The two proteins function antagonistically, with Bax promoting cell death (pro-apoptotic) and Bcl-2 inhibiting cell death (anti-apoptotic) by inhibiting Bax activity. Due to their relationship, Bcl-2 and Bax levels are often referred to as ratios of Bcl-2/Bax. [2]
Exercise: Bcl-2/Bax Ratios
Caspase-3 belongs to the family of caspases, which all play a role in cell apoptosis. In order to be activated to cause the downstream effects that lead to apoptosis, caspase-3 needs to be cleaved. When a cell expresses cleaved caspase-3, this would suggest the cell is undergoing apoptosis.[3]
Results
Using western blot, researchers found that vitexin had a dose-dependent effect on the expression of pro-apoptotic proteins in A549 cells. All tested doses of vitexin decreased the ratio of Bcl-2/Bax proteins, suggesting either a decrease in the anti-apoptotic Bcl-2, an increase in the pro-apoptotic Bax, or both. Additionally, levels of cleaved caspase-3 increased significantly with the treatment of vitexin. This relationship once against showed a dose-dependent effect.
Experiment Overview
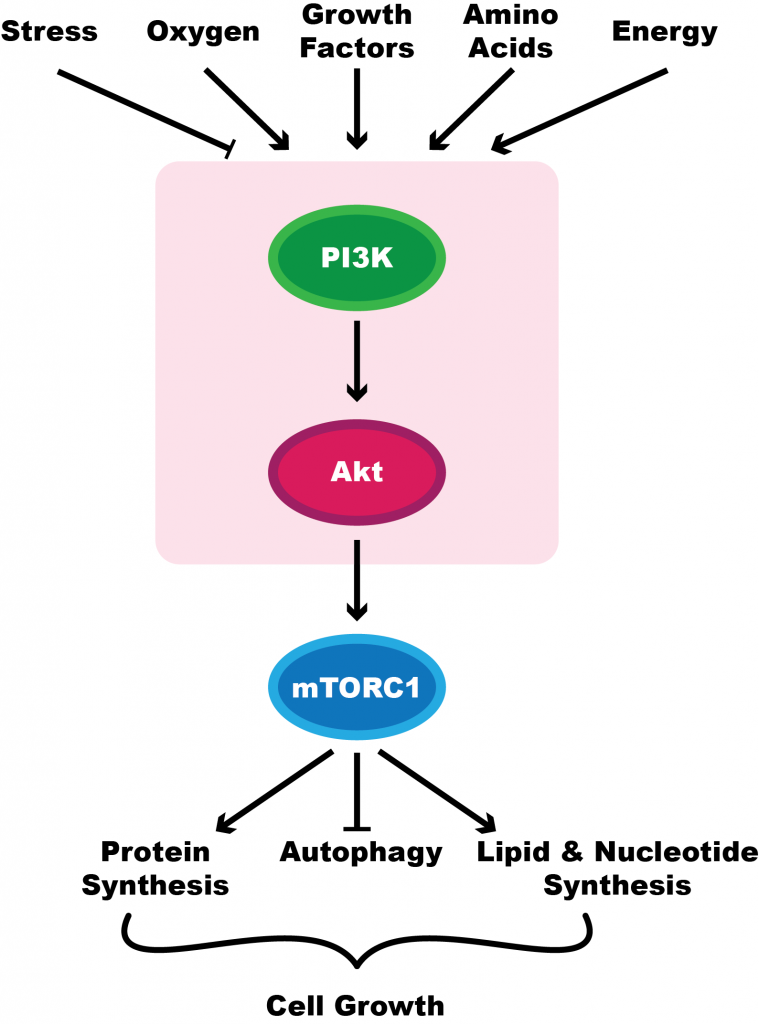
The mammalian target of rapamycin (mTOR) signaling pathway plays a crucial role in regulating aspects of the cell cycle. This includes cell metabolism and growth. The pathway has been shown to be activated in cancer processes, including during tumour formation and angiogenesis.
Due to the role that mTOR plays in cancer proliferation, Liu et al. (2019) ran an experiment to determine if vitexin treatment had any effect on this pathway, potentially explaining the anti-tumour effects their past experiments had shown. Additionally, the mTOR signaling pathway has been a target for different cancers in the past.[4][5]
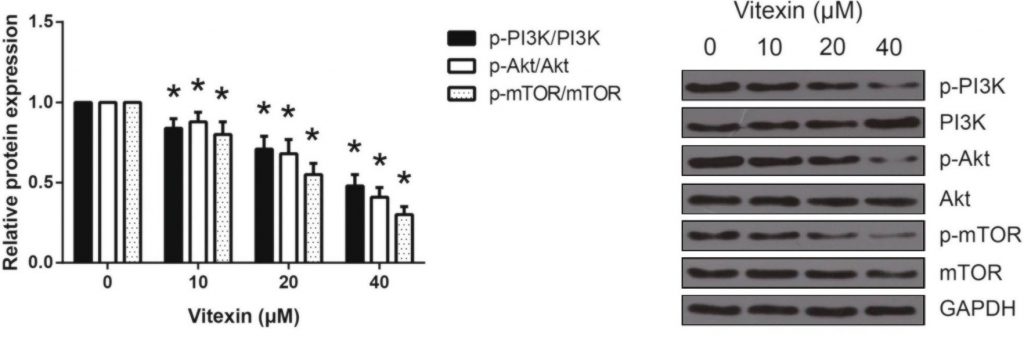
The researchers ran western blot analysis for p-PI3K, p-Akt, and p-mTOR, all of which are involved in the signaling pathway. The greater the expression of these proteins, the more cell growth will occur.
Results
Using western blot analysis, researchers found that treating A549 cells with vitexin led to significantly lower expression of the main proteins involved in the mTOR pathway, including PI3K, Akt, and mTOR. The results in figure 4 highlight the statistically significant decrease even with the lowest dose of vitexin. The additional decrease as the dosage increases suggests the effects are dose-dependent.
Experiment Overview
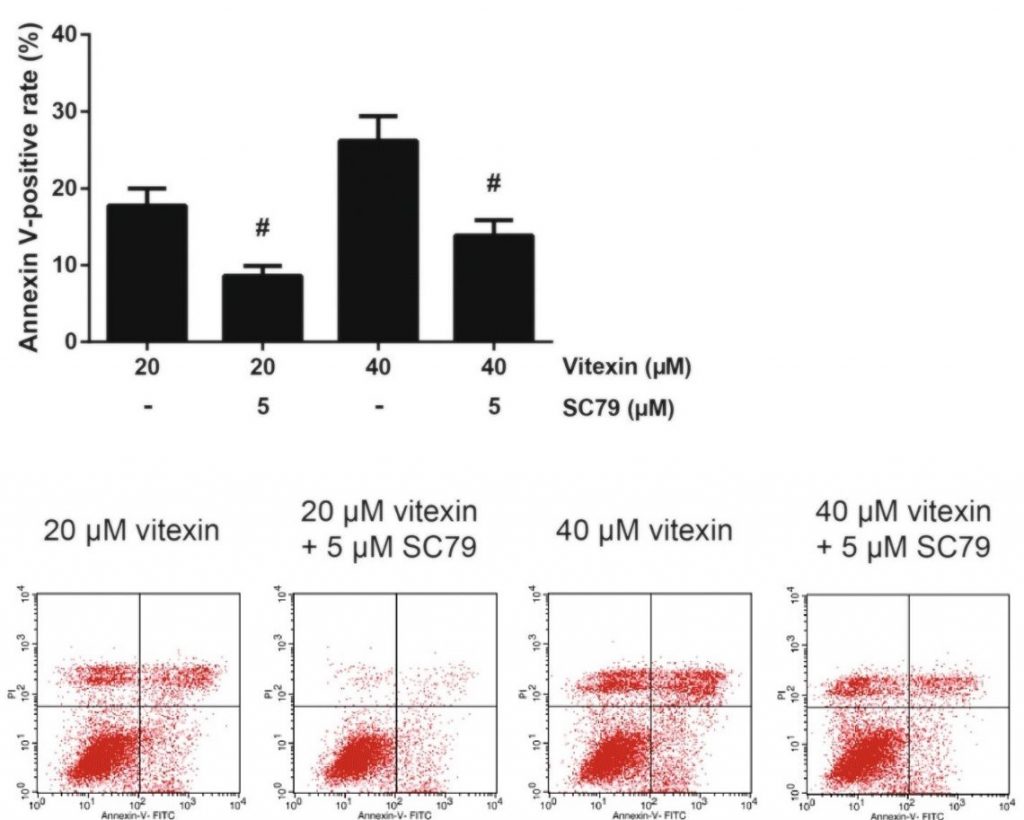
With vitexin having an effect on the mTOR pathway, the researchers would still be required to know if the changes to the pathway are responsible for the pro-apoptotic effects seen in previous experiments. To do so, the researchers also introduced an Akt activator, SC79, to cells treated with vitexin. If apoptosis is reduced in the presence of Akt activation, then it would indicate that the effects of vitexin stem from this pathway.
The researchers ran a similar annexin V-FITC/PI flow cytometry experiment to determine if SC79 treatment could reverse the apoptotic effects of vitexin.
Results
After treatment with the Akt activator, SC79, researchers found a reversal of the apoptotic effects that vitexin had on A549 cells. Treatment of cells with 5 μM of SC79 reduced the proportion of annexin V-positive A549 cells by a statistically significant amount. The results of the experiment are shown in Figure 5.
Summary
Western blot analysis was used the researchers in order to determine the following:
- The expression of apoptotic proteins in A549 cells treated with vitexin.
- The levels of proteins involved in the mTOR pathway in A549 cells treated with vitexin.
Researchers also reversed the apoptotic effects of vitexin on A549 cells by treating the cells with SC79, an activator of Akt, a protein in the mTOR pathway.
- Mahmood T, Yang P-C. 2012. Western Blot: Technique, Theory, and Trouble Shooting. N Am J Med Sci. 4(9):429–434. doi:10.4103/1947-2714.100998. ↵
- Khodapasand E, Jafarzadeh N, Farrokhi F, Kamalidehghan B, Houshmand M. 2015. Is Bax/Bcl-2 Ratio Considered as a Prognostic Marker with Age and Tumor Location in Colorectal Cancer? Iran Biomed J. 19(2):69–75. doi:10.6091/ibj.1366.2015. ↵
- McIlwain DR, Berger T, Mak TW. 2013. Caspase Functions in Cell Death and Disease. Cold Spring Harbor Perspectives in Biology. 5(4):a008656–a008656. doi:10.1101/cshperspect.a008656. ↵
- Laplante M, Sabatini DM. 2009. mTOR signaling at a glance. Journal of Cell Science. 122(20):3589–3594. doi:10.1242/jcs.051011. ↵
- Saxton RA, Sabatini DM. 2017. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 168(6):960–976. doi:10.1016/j.cell.2017.02.004. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5394987/ ↵
The formation of new blood vessels. In cancer, these new blood vessels provide a way for cancer cells to travel to other parts of the body.
