50 6.3 The Use of Atorvastatin on Chronic Kidney Disease (CKD) Patients
Learning Objectives
In this chapter, you will learn about:
- An experimental study using the atorvastatin drug to treat patients with CKD
- Methods used to conduct an ASUCA trial
Experimental Overview of the Study by Kimura et al. (2017)
Atorvastatin has been widely used to treat dyslipidemia in patients with cardiovascular disease. Because dyslipidemia is also a major health complication and risk factor of chronic kidney disease (CKD), scientists are currently investigating whether this type of statin drug can help improve the renal function of patients with chronic kidney disease. A study conducted by Kimura et al tries to examine the potential protective effects that atorvastatin could have on impaired kidney function.
Experimental procedure:
From the study, the researchers conducted a large-scale clinical study called the assessment of clinical usefulness in CKD patients with atorvastatin, which is also known as the ASUCA trial. The ASUCA trial is a randomized controlled study done in Japan. In the study, approximately 349 Japanese patients were screened for eligibility and were assessed for a specific criteria (Kimura et al., 2017).
- The cohorts had to be 40-75 years of age
- They should not have been previously treated with statin drugs
- Required to have proteinuria and glomerular filtration rates of less than 60 ml/min/1.73 m² and LDL levels less than 140 mg/dl for those who are taking dyslipidemic drugs other than statin drugs
The study also included an exclusion criteria where they omitted patients from the study who:
1) had glomerular filtration rates of less than 30 ml/min/1.73 m²
2) had systolic and diastolic blood pressure levels lower than 110 and 180 mmHg respectively
3) have liver dysfunction problems such as hepatitis, and liver cirrhosis, as well as patients with past experience with severe side effects to the statin drug and pregnant women from the trial.
After the researchers assessed the patients for eligibility, the patients were randomly assigned into two groups : Group A and Group C
- Patients in group A were given the atorvastatin drug and were assigned to a diet therapy. The dose of the drug was 10 mg/day and was increased to 5-20 mg/per day. If the LDL-C levels did not decrease to the target level (which was 100 mg/dl) after 3 months, then anti-dyslipidemic drugs were given (Kimura et al., 2017).
- For group C ,which was the control group, patients were assigned a diet therapy but with no atorvastatin treatment. If the drug treatment in group C failed to lower the LDL-C levels to 100 mg/dl, the patients would be administered with lipid lowering drugs that are not statins drugs such as ezetimibe. Ezetimibe is a conventional drug that lowers the level of lipids and lipoproteins, and it’s often used to reduce the level of cholesterol in the blood (Kimura et al., 2017).
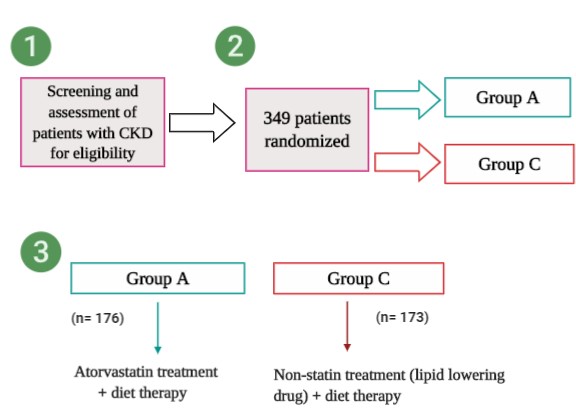
Fig.2 Diagram of the study protocol (adapted from Kimura et al, 2017 using Biorender).
- To randomly stratify the population, they considered gender, hypertension, diabetes mellitus, and treatment with renin angiotensin aldosterone system (RAAS) inhibitors in their baseline measurements (Kimura et al., 2017).
For primary outcomes: The scientists measured changes in the eGFR (glomerular filtration rate) between the two groups.
For secondary outcomes: The differences of albumin and creatinine in urine, changes in LDL, and serum triglyceride levels were measured. They accounted for severe cardiovascular events, which included any potential death and hospitalization after use of the atorvastatin drug. In addition, the measurements for these variables were determined through the run of laboratory tests where the patients’ blood was collected. The consistent data collection for the study was conducted prior to the treatments and on day 1,3,6,9,12,18 and 24 months after the procedure started (Kimura et al., 2017). Overall, this clinical study lasted for 2 years.
Test Your Knowledge
