34 2.7: Endophilin-1 Knockdown
Quick Overview
As stated in section 2.6, endophilin-1 is involved in multiple parts of the endocytosis pathway on clathrin-coated vesicles, which is relevant to Alzheimer’s disease because this pathway is prominent in
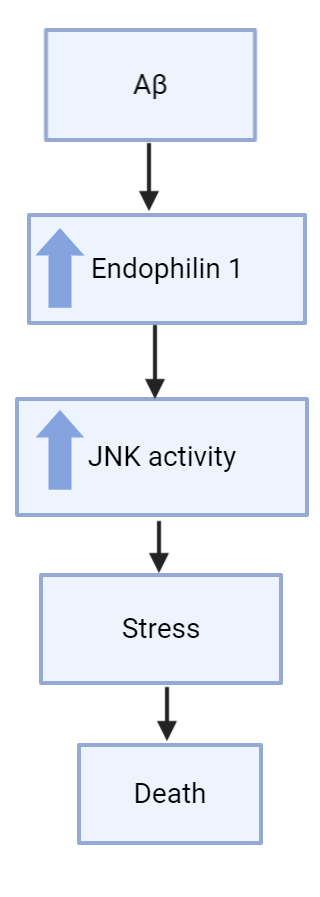
presynaptic terminals in the brain, which can affect long-term potentiation and long-term depression (Reutens 2002). Endophilin-1 is also interacts with c-Jun N-terminal kinase (JNK), which is part of the mitogen-activated protein kinase (MAPK) pathway that is responsible for various cellular processes like cell proliferation and apoptosis (Reutens 2002). JNK in the MAPK pathway usually leads to apoptosis, so endophilin-1 in this situation likely contributes to this apoptosis in some manner.
The relevance of Endophilin-1 to Alzheimer’s disease comes into play when we look at hippocampal neurons treated with Aβ1-42 oligomers. A study found that endophilin-1 in hippocampal neurons was highly expressed when the neurons had been treated with Aβ1-42 oligomers and underwent synaptic dysfunction (Yin et al, 2019). The more Aβ1-42 oligomers the neurons were exposed to, the more synaptic dysfunction (lower frequency and amplitude of action potential), and the higher the expression of endophilin-1. This left the researchers wondering if endophilin-1 somehow plays a role in causing Alzheimer’s disease.
Endophilin-1 & Alzheimer’s Disease
With the results indicating endophilin-1 played a part with Aβ1-42 oligomers in neuronal death and dysfunction, the researchers wanted to see if knocking out endophilin-1 could be a possible treatment for Alzherimer’s disease, especially for relieving symptoms like synaptic dysfunction that leads to long-term depression. Though lowering synaptic dysfunction and increasing long-term potentiation is not a cure, it could potentially improve patients quality of life greatly by allowing them some new memory retention. When using a plasmid to block endophilin-1 production, hippocampal neurons that were treated with Aβ1-42 oligomers showed increased amplitude and frequency in their miniature excitatory synaptic currents (MEPSCs (recorded by whole-cell patch clamp technique)) compared to hippocampal neurons that were just treated with Aβ1-42 oligomers and no endophilin-1 knock out (Yin et al, 2019). This is a good start, but other studies also using hippocampal neurons and patch-clamp recording found that knocking out endophilin-1 had no effect on the intrinsic electrophysiology of neurons (Zhang et al, 2015). Though this study was a bit different, this shows that more studies are needed to confirm this potential connection. One possible way to do this could be by using in vivo mouse models instead of in vitro hippocampal neurons to possibly yield more conclusive and applicable results.
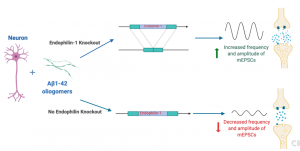
A Possible Endophilin-1 Pathway
The answer could possibly be related to AMPA receptors. AMPA receptors are responsible for
lots of excitatory signalling in the brain via ion channels (Lee et al, 2002) and they also undergo clathrin-dependent endocytosis (Chowhury et al, 2006). When Aβ1-42 oligomers concentration is increased in hippocampal neurons, endophilin-1 expression was also increased, which led to an increase in synaptic dysfunction and long-term depression(Yin et al, 2019). Though no mechanism was directly described in this study, other studies have found that an increase in Aβ1-42 oligomers by overexpression of amyloid precursor protein led to synaptic depression as well due to endocytosis of AMPA receptors (Hsieh et al, 2006). Since AMPA receptors undergo clathrin-dependent endocytosis, and endophilin-1 is involved in this clathrin-dependent endocytosis pathway, it is possible that increased amounts of Aβ1-42 oligomers lead to increased expression of endophilin-1 and causes increased endocytosis of AMPA receptors, preventing them from firing excitatory signals and causing synaptic depression in the neurons, a cause of Alzheimer’s disease.

Another possible explanation of the pathway could involve a protein called Arc/Arg3. In a study by Chowdhury et al. (2006), they show that a protein called Arc/Arg3.1 interacts with dynamin and endophilin-2 and endophilin-3 to increase AMPA receptor endocytosis. In a study done by Zhang et al. (2017), it was demonstrated that the knockdown of endophilin-2 resisted the synaptic dysfunction mediated by AMPA receptor endocytosis caused by amyloid-β. The knockdown of endolphilin-1 shown similar results, as synaptic dysfunction caused by amyloid-β was prevented (Yin et al., 2019). Endophilin-1 functioning similarly as endophilin-2 is thought to also be involved in the endocytosis of AMPA receptors caused by amyloid-β. These are potential targets which could be used for treatment of Alzheimer’s disease.
