32 2.5: Whole-Cell Patch Clamp Technique
What is it?
The patch clamp technique, a discovery made in 1976, allowed the study of single or multiple ion channels in living in vitro cells (Suk et al, 2019). Later, the whole-cell patch clamp technique was invented, which allowed for the study of electrical neuron activity as well as ion channels (Segev et al, 2016). It was first discovered and used on in vitro cells, but more recently it has been used in vivo as well (Suk et al, 2019). It was originally developed as a way to study ion channels more closely and to get accurate, high-resolution recordings (Wallis, 1993). For both in vitro and in vivo techniques, a glass micropipette tip is surrounded by a microelectrode and both are bathed in an intracellular solution similar to that of the cytosol of the cell (Jonas et al, 1997). The electrode is placed on the cell membrane and gentle suction from the pipette is used to break the cell membrane (Wallis, 1993). When the break in the cell membrane is made, a ‘patch’ is formed and a gigaohm seal is created, which is the essential step in this technique. This gigaohm seal allows the pipette to act as part of the membrane, reducing the leakage current of the hole made in the cell membrane and allowing the electrode placed in the pipette to record the current and the action potentials of the cell (Wallis, 1993). The neural activity recordings are taken via an amplifier attached to the electrode, and this is sent to a computer system to be analyzed by various computer programs, depending on what the researcher is studying. In vitro and in vivo patch clamp techniques differ mainly in the preparation of the cells or brain tissue, with the actual neural activity recording technique being quite similar (Suk et al, 2019).
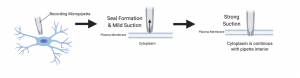
Advantages
- Can be used on neurons in various preparations (Segev et al.)
- Can yield real-time and fast results
- Easy to add drugs or ions to cells to see a reaction
- Can be used for experiments on various cells in the body
Disadvantages
- Very difficult and very technical
- It is a costly technique with the need for specialized equipment
- Easy to rupture the cell membrane
- Computer analysis could be flawed
Importance to Alzheimer’s Disease
When studying Alzheimer’s Disease, a very important aspect is the ability of neurons to fire and synaptic function. As we mentioned in section 2.4, synaptic dysfunction via oligomer Amyloid-β 1-42 (Aβ1-42) can lead to neuronal death as well as impair neurons’ abilities to fire, increasing long-term depression that affects learning and memory (Yin et al.). This indicates that the ability to study neuronal firing is essential to understanding the way Alzheimer’s disease and Aβ1-42 oligomers affect our brain function.
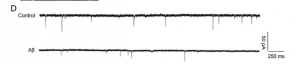
The whole-cell patch clamp technique is a way to study neurons ability to fire, and allows us to manipulate the neurons with proteins known to contribute to Alzheimer’s disease, like Aβ1-42 oligomers or tau proteins. It shows how
the neurons respond and whether their synaptic function is affected, which could indicate long-term potentiation is affected, which is indicative of Alzheimer’s disease.
In the study by Yin et al., they used this technique on neurons exposed to Aβ1-42 oligomers and cells with endophilin-1 knocked out (as well as control). They discovered that knocking out endophilin-1 increased the amplitude and frequency of a neurons miniature excitatory synaptic current compared to the control, where exposure to Aβ1-42 oligomers increased endophilin-1 expression, creating synaptic dysfunction. Future directions could be repeating the experiment in vivo using rats or mice (rather than in vitro on hippocampal neurons), and seeing if the results hold up.
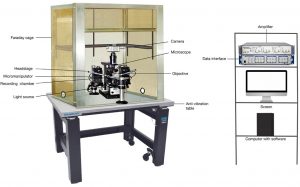
Overall Importance
The whole-cell patch clamp technique is not only used for studies of the nervous system. Its ability to record ion channel levels and electrical activity is very helpful throughout the whole body, either for information regarding physiology or helping us understand how drugs could affect ion channels in the body. It is very broad, from being used to measure the Na+/K+ -ATPase pumps in the epithelia of the lens and ciliary body cells in the eyes; as well as funny channels (If channels) and pacemaker current in sinoatrial node cells of the heart(Wallis, 1993). More recently, a study by Ossola et al. has used this technique to study voltage-gated ion channels in actively beating cardiomyocytes. This technique is very useful for study in almost all parts and cellular processes of the body.
Quick Fact!
It is also very helpful for pharmaceutical studies, as the internal pipette solution is able to diffuse drugs or other substances directly into the cytoplasm of the neuron! This allows for real-time observation of the effects the drug has on the function of the cell and its ion channels (Segev et al.).
