41 4.1 Introduction
Overview of Myocardial Infarction
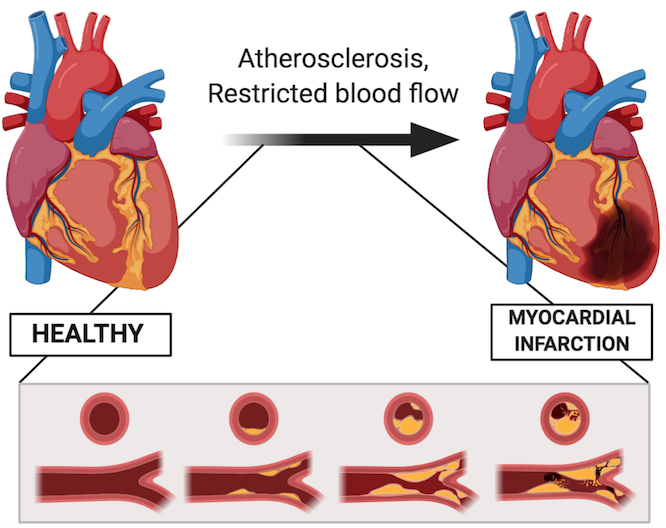
Cardiovascular disease is the leading cause of death around the world. Specifically, coronary artery disease, which is caused by atherosclerosis – a build of plaques in your coronary arteries. Atherosclerosis then leads to ischemia and oxidative stress, resulting in inflammation and necrosis of the surrounding tissues (Hori & Nishida, 2009). Consequently, myocardial infarction, or what is more commonly known as a heart attack, can occur. Myocardial infarction refers to the lack of blood flowing to the myocardium, leading to tissue death (McLaughlin et al., 2019). As a result, cardiac function is often negatively affected.
Current therapies for myocardial infarction such as drugs, growth factors and cell-based therapies are sub-optimal at restoring cardiac function (McLaughlin et al., 2019). Surgical procedures focus on restoring blood flow, but do not restore function. This is important because approximately 10% of patients develop adverse ventricular remodelling following a heart attack, increasing their risk of heart failure (Horn & Trafford, 2016).
Researchers have identified two main factors that interfere with the long-term restoration of heart function. The first is the limited endogenous ability of cardiomyocytes to heal due to their post-mitotic nature (Anversa, Leri, Kajstura, & Nadal-Ginard, 2002). Unlike nerve cells or blood cells that can regenerate and repopulate, heart cells are unable to do so. This is why it is so important to protect your heart and keep it healthy! The second factor is due to the changes in the extracellular matrix that occurs during myocardial infarction. This chapter will take an in-depth look into the second factor.

The Extracellular Matrix and The Heart
The extracellular matrix (ECM) is composed of a variety of proteins and carbohydrates responsible for structural support (Hughes & Jacobs, 2017). The ECM also facilitates cell-ECM interactions that are important for cell signalling, function, and survival (Heino, 2000). In the heart, structural support is primarily provided by types I and III collagens, contributing to the stability and tensile strength of the myocardium (Hughes & Jacobs, 2017). This has significant implications in cardiac function, as the myocardium allows the heart to pump blood efficiently throughout your body. When an individual experiences myocardial infarction, the ECM undergoes remodelling (Figure 3B) which can lead to adverse ventricular remodelling, and consequently, impaired cardiac function (Nielsen et al., 2019). Thus, targeting ECM remodelling during the proliferative phase post-MI is a promising target for MI therapies, in order to limit adverse remodeling and the loss of cardiac function (McLaughlin et al., 2019).
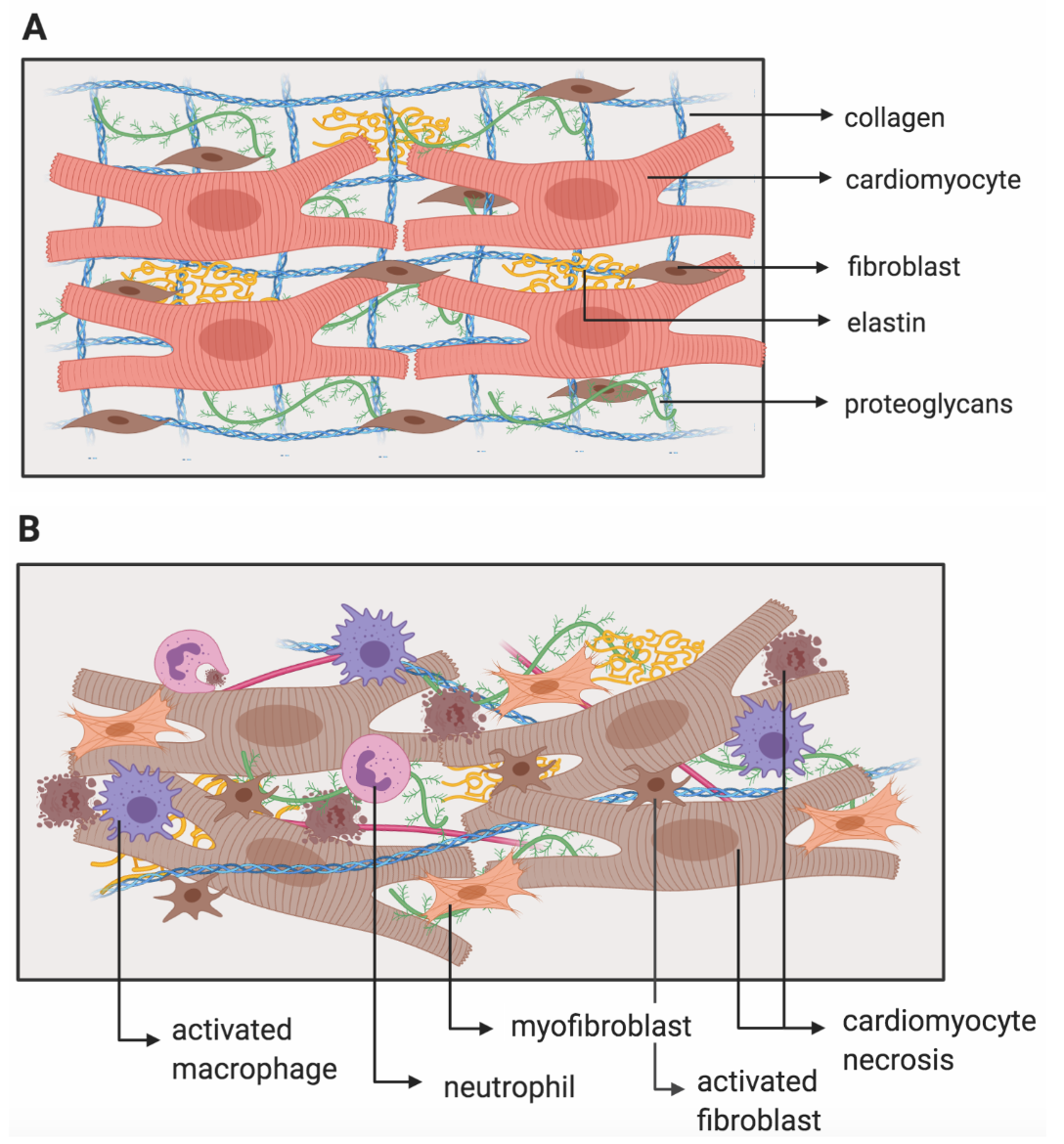
In the past, studies conducted on animal models of MI using collagen-based biomaterials have shown an increase in mechanical support and angiogenesis, and a decrease in inflammation (Rane & Christman, 2011). However, these studies used collagen of animal-origin and therefore lack translationality to humans. This is because of the inherent immune risks of endotoxins, and batch-to-batch variability that exists in these studies (McLaughlin et al., 2019). In addition, these studies focused more on the late-proliferative to early maturation phases and not on earlier stages post-MI.
Injectable human collagen can potentially mimic endogenous collagen matrices and promote infarct repair (McLaughlin et al., 2019). This would function as a long-term treatment as well as present potential healthcare savings for those who experience myocardial infarction. It is clinically translatable as it originates from human sources, and it has been safely used as a therapy during corneal implants (Fagerholm et al., 2010). A study by Mclaughlin et al. used recombinant human collagen (rHC) and targeted the early proliferative post-MI phase (2019). This phase acts as a model for those patients who don’t get treatment right away – sometimes people don’t recognize the signs of a heart attack or have easy access to medical attention. In the following sections, this study will be analyzed and examined in greater detail.
Key Takeaways
- What is myocardial infarction?
- What is the role of the ECM in cardiac function?
- Why use recombinant human collagen as a treatment for MI?
- Why is it significant that McLaughlin et al. target the early proliferative post-MI phase?
