4 Osmoregulation
To function properly, the cellular machinery needs just the right amount of water and solutes. Unfortunately, solutes and water move in and out of cells and the body following concentration and osmotic gradients. All living organisms must thus actively regulate the perpetual movement of solutes and water across biological membranes. The regulation of water and solutes go hand in hand and are collectively called osmoregulation. Osmoregulation is particularly interesting in amphibians and reptiles because both groups face major, but different, osmoregulatory challenges. As we’ve seen, amphibians have a thin and permeable skin, a requirement for cutaneous respiration. Water and solutes however readily move through the skin of amphibians creating a conflict between cutaneous respiration and osmoregulation. Reptiles, in contrast, do not heavily rely on cutaneous respiration (aside from a few aquatic species of snakes and turtles) and their skin is covered by scales that are rich in hydrophobic lipids creating an quasi-impermeable barrier. Scales are one of many omsoregulatory adaptations allowing reptiles to thrive in the most hot and arid ecosystems on the planet.
Water Budget

To keep their internal water content within its physiological sweet spots, animals must constantly satisfy this simple equation: water gain = water loss.
In this section we will look at both sides of this equation and discuss how amphibians and reptiles use morphological, behavioural, and physiological adaptations to balance it.
Water can enter the body of animals in three forms
- Liquid water (absorbed by the skin or drank)
- Preformed water (food)
- Metabolic water (by-product of metabolism)
Once in the body, water can be stored in the urinary bladder as well as in intracellular and extracellular fluids.
Liquid Water
Liquid water is obtained directly from the environment. The most familiar way to obtain liquid water is to drink it. Terrestrial reptiles drink from pools, puddles, or rain drops. In deserts, some reptiles harvest rain water with their body and direct the water to their mouth. For instance, some rattlesnakes tighten their coils and flatten their body during rain showers to form a rain-harvesting dish of sort. They then drink from the puddle accumulating on their body. This behaviour is accompanied by morphological adaptions of the Oberhautchen. The surface of the scales of the diamondback rattlesnake is covered by an elaborate network of micro-channels that pin down rain droplets so that they remain on the skin rather than spilling to the sides. This video shows the rain harvesting behaviour if the western diamondback rattlesnake (Crotalus atrox) and summarizes how the surface of the skin helps retain droplets.


Horned lizards (Phrynosoma sp) have similar behavioural and morphological adaptations to harvest rain. The surface of their scales bears microscopic honeycomb-like ornamentation which render their scales super-hydrophilic. The ornamentation also promote the condensation of water vapour on the skin. Indeed, the ornamented scales of horned lizards condense twice as much water as a flat surface. Horned lizards can thus harvest water vapour with their body. The water is then passively transported to the mouth via capillary action thanks to microscopic channels on the skin surface. Interestingly, the channels somehow move the water more readily towards the mouth than away from it (see image below). When it rains, horned lizards adopt a rain harvesting posture by elevating their hindquarters and lowering their head which directs rain drops to their mouth.

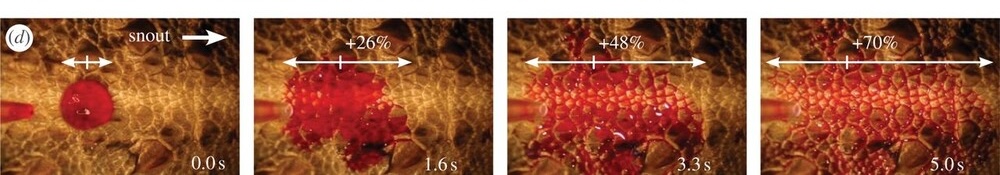
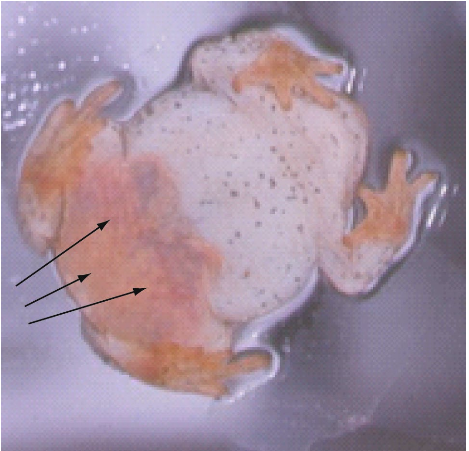
Amphibians don’t drink! They get all the water they need from their food (preformed water) or they absorb it through their skin. Many terrestrial anurans have a patch of thin and densely vascularized skin in the pelvic area which is called the pelvic patch or seat patch. This area absorbs water from puddles or from the moist substrate.
The water is then stored in a huge bladder. When filled, the bladder of terrestrial amphibians can make up 20 to 60% of their body weight. For comparison, a filled human bladder makes-up less than 0.01% of the body weight. The bladder stores a large amount of diluted urine that amphibians can tap into when water is scarce. The solute concentration of their urine is low compared to that of the plasma which favours the movement of fluids from the bladder to the plasma. Desert anurans such as spadefoot toads (Spea and Scaphiopus) can survive for months underground absorbing water directly from the surrounding dry soil. They achieve this feat by elevating the solute concentration of their plasma. As you know, water moves from areas of high solute concentration to areas of low solute concentration. Elevating the solute concentration of the plasma favours the movement of water from the soil to the body.

Freshwater amphibians face a different osmoregulatory challenge than their terrestrial counterparts. The solute concentration in freshwater is much lower (about 1 – 2 mmol/Kg of water) than the internal milieu of the animal (about 250 mmol/Kg of water). Combine this steep osmotic gradient with the high permeability of amphibian skin, and you get a net influx of water into the body and a net loss of ions from the body. High water influx is not a problem because water can rapidly be eliminated in the surrounding water as diluted urine. In other words, aquatic amphibians pee a lot. Freshwater amphibians thus do not need to store a lot of water and thus have relatively small bladders (1 to 5% of body weight). The loss of ions from the body is however a more serious issue. Aquatic amphibians must continually pump the ions escaping their body back into their body. Pumping these ions back has a cost in the form of ATP. Active ion uptake occurs through the gills (when present) and the skin. Pumping ions is essential but it become a liability when the solute concentration of the aquatic environment increases. A major source of concern for amphibians in temperate areas is the increase salinity of wetlands resulting from the widespread application of road salt. In a saline environment, solutes enter the body instead of leaving it, and water leaves the body instead of entering it. In essence, the fluxes of water and ions are reversed. This throws the internal environment of amphibians off kilter and more ATP is used to excrete excessive solutes while water is passively lost to the environment. As a result, freshwater amphibians exposed to saline water, grow and reproduce at a slower rate than those under normal conditions.
Preformed Water
Most amphibians and reptiles are predators. Animal tissues typically contain 70 to 80% water. The water content of plants is much more variable between species and between seasons.

Digesting a meal requires water, and, in some extreme cases, the meal may contain less water than is required to digest it. Chuckwallas (Sauromalus obesus), a medium size lizard of the Sonora and Mojave deserts of the North American Southwest, must contend with this issue. Chuckwallas are herbivorous and get most of their water from the plants they eat. During the dry season, the water content of plant drops. If it falls below 70%, Chuckwallas stop eating because the plants they ingest contain less water than it takes to digest them. Feeding would thus lead to dehydration. Digesting certain prey items may also lead to a net water loss. Gila monsters (Heloderma suspectum) and some sea snakes loose more water digesting their prey than they gain from them and thus must obtain water from other sources than their food.

Metabolic Water
Water and carbon dioxide are by-products of the oxidation of food. Lipids, proteins, and carbohydrates yield different amount of metabolic water. The net amount of metabolic water derived from these substrates depends on both the amount of water a unit of substrate can yield and the amount of oxygen required to oxidize that substrate. For instance, lipids yield more water per gram of substrate than proteins and carbohydrates, but a gram of lipids also requires more oxygen to be oxidized. The oxygen used to oxidize food comes from the ventilation of respiratory surfaces. These surfaces must be moist for oxygen to diffuse through them and into the blood stream. The rate of water loss over respiratory surfaces is proportional to the ventilation rate, so increasing the ventilation rate increases water loss. Taking the respiratory water loss into account, carbohydrates yield more metabolic water than proteins and lipids per gram of substrate.

Metabolic water is a negligible source of water in amphibians because their metabolism produces little water compared to their high rate of cutaneous water loss (see below). Reptiles require roughly 1 to 5% of the water amphibians need so metabolic water is relatively more important for reptiles. Shoemaker and Nagy (1977) estimated that small, diurnal, desert dwelling reptiles obtain 10 to 20% of their water via their metabolism.
Water Loss
Water leaves the body primarily through three routes
-
- Respiration
- Evaporation
- Excretion
Respiration
To enter the blood stream, oxygen must first be dissolved in a fluid. In most tetrapods this happens on the wet surface of the lungs. To do their job, the surface of the lungs must be wet. Lungs have a large surface area which is a necessity for gas exchange so lung ventilation can be a major source of water loss. Water loss from the lungs depends on the ventilation rate. The higher the ventilation rate, the higher the water loss. Endotherms have much higher ventilation rates than ectotherms because of their higher metabolic rate. Birds and mammals minimize respiratory water loss by condensing and reabsorbing the water that evaporated from the lungs during ventilation. This happens in the nasal turbinates just before the exhaled air leaves the body. Amphibians and reptiles lack such nasal water-condensating hardware presumably because the loss of water from lung ventilation is insignificant due to their relatively low metabolism. Few studies have quantified respiratory water loss in reptiles. Mautz (1982) and Crawford and Kempe (1982) found respiratory water loss to account to 50% of the total water loss in Night Lizards (Xantusiidae) and Chuckwallas (Sauromalus obesus). In contrast, Thompson and Withers (1997) found respiratory water loss to account for a maximum of only 6% of the total water loss in small varanid (i.e. monitors) lizards. Lung ventilation is probably a negligible route of water in amphibians because they lose important quantities of water through their skin (see below).
Evaporation
The skin is the most important source of evaporative water loss in amphibians and reptiles because to it has a large surface area and it is in direct contact with the environment. The rate of cutaneous water loss depends on morphological, behavioral, and environmental factors.
Cutaneous water loss occurs in all amphibians and reptiles, but it is more important in terrestrial amphibians because the skin also an important site for gas exchange. The skin of terrestrial reptiles is relatively impermeable thanks to the hydrophobic lipids found in their scales.
To function as a gas exchange surface, the skin must be thin and permeable. This creates a conflict between water conservation and gas exchange. Both amphibians and reptiles can breathe through their skin, but cutaneous respiration in reptiles is largely restricted to aquatic species for which losing water through the skin is not an issue. In contrast, many terrestrial amphibians rely on their skin for much of their gas exchange. Those amphibians must carefully balance respiration and water loss. The need to minimize water loss dictates many aspects of the ecology and behaviour of terrestrial amphibians.
How rapidly an animal loses water through its skin is measured as the time it takes for water to diffuse through one centimetre of skin. This number is called the cutaneous resistance. An open water surface has a resistance of 0 s/cm. Cutaneous resistance varies greatly among amphibians and reptiles. From 0.05 s/cm in aquatic amphibians to over 1000 s/cm in desert dwelling reptiles. For reference, cutaneous resistance in mammals varies between 200 and 400 s/cm.
Cutaneous water loss is particularly acute in terrestrial plethodontid salamanders. These salamanders lack lungs and rely almost exclusively on their skin for their respiration. The resistance of their skin to water loss is close to 0 s/cm. In other words, water evaporates through the skin of these salamanders at the same rate as from an open dish of water. Such high rates of water loss limits where and when these salamanders can be active. For instance, Peterman and Semslitch (2014) found that Western Slimy Salamanders (Plethodon albagula) were more abundant and active, and had a higher reproductive success in forested areas with micro-climates minimizing evaporative water loss (cooler and wetter).
Most amphibians are nocturnal. This habit may be linked to osmoregulation because the air temperature is cooler and the relative humidity higher at night. Whether nocturnality evolved primarily to minimize water loss is unclear but being active at night surely has osmoregulatory benefits. During the day, terrestrial amphibians often hide in cool and wet refuges. Some tree frogs, like metamorphic gray tree frogs remain exposed to the sun and wind during the day, but they typically adopt a water conservation posture by tucking their limbs under their body.

Tree frogs (family Hylidae) tend to have higher cutaneous resistance than other amphibians which is presumably an adaptation to living in trees where evaporation by convection from the wind is higher than on the ground. For instance, in a study on spring peepers Cicchino et al. (2020) found that the rate of evaporative water loss was 5 times higher 1.2 meter above the ground than 5cm above the ground. This study also suggests that spring peepers face a trade-off between evaporation water loss and call broadcasting when selecting a calling sites. Higher calling perches broadcast the sound further but lead to more rapid dehydration.
Certain tree frogs like the waxy leaf frogs further reduce water loss by secreting a lipid-rich waterproofing cocktail that they smear over their body with their limbs. This coating brings up their skin resistance to 900 s/cm which is similar to a desert reptile. With this coat on, waxy leaf frogs can rest on branches fully exposed to the sun and wind.

Some frogs and salamanders cope with extended dry spells by forming a cocoon made up of layers of shed skins or mucus. The cocoon can have a water loss resistance 50 times higher than skin

Excretion
Water is essential to rid the body of metabolic wastes. Proteins obtained in food are broken down into amino acids that are used to build new proteins. The de-amination of proteins in the liver produces ammonia. Ammonia is highly toxic, and cannot be stored without further transformation so it must be excreted rapidly. Thankfully, ammonia is soluble and readily diffuses across the skin and gills. Vertebrates evolved in water and could thus afford to excrete large quantities of water along with ammonia. Aquatic amphibians retain this ancestral mode of excretion. Animals excreting primarily ammonia are called ammonotelic.

Ammonotely is only an option for aquatic amphibians because ammonia must be excreted with a copious amount of water. Terrestrial amphibians excreting ammonia would thus rapidly suffer from dehydration. Terrestrial and semi-aquatic herps transform ammonia into less toxic forms that can be stored and excreted with less water than ammonia but this water-saving strategy is not free.
Ammonia can be transformed into urea at the cost of 4 moles of ATP per mole of urea. Urea is less toxic than ammonia so it can be stored safely. It is also more soluble than ammonia so more waste can be excreted per drop of water than with ammonia. Most terrestrial amphibians and freshwater turtles excrete nitrogenous waste in the form of urea. They are ureotelic.
Reptiles and a few arboreal frogs have managed to excrete even more waste per drop of water by converting ammonia into uric acid but at the whopping cost of 24 ATP per mole of uric acid. Uric acid has the advantage of being insoluble so it can be excreted as a semisolid. Uric acid precipitates into crystals as water gets reabsorbed in the bladder or cloaca. Animals excreting uric acid are called uricotelic.
References
Abdel-Aal, H., El Mansori, M., & Zahouani, H. (2017).A comparative study of frictional response of shed snakeskin and human skin. Wear, 376–377, 281–294. https://doi.org/10.1016/j.wear.2016.12.055
Cardwell, M. (2006). Rain-harvesting in a wild population of crotalus s. scutulatus (serpentes: Viperidae). Herpetological Review, 37, 142.
Cartledge, V. A., Withers, P. C., McMaster, K. A., Thompson, G. G., & Bradshaw, S. D. (2006). Water balance of field-excavated aestivating Australian desert frogs, the cocoon-forming Neobatrachus aquilonius and the non-cocooning Notaden nichollsi (Amphibia: Myobatrachidae). Journal of Experimental Biology, 209(17), 3309–3321. https://doi.org/10.1242/jeb.02393
Comanns, Philipp & Buchberger, Gerda & Buchsbaum, Andreas & Baumgartner, Richard & Kogler, Alexander & Bauer, Siegfried & Baumgartner, Werner. (2015). Directional, passive liquid transport: The Texas horned lizard as a model for a biomimetic ‘liquiddiode’. Journal of the Royal Society, Interface / the Royal Society. 12. 10.1098/rsif.2015.0415.
Cicchino, AS, NC Cairns, G. Bulté, & SL Lougheed. (2019). High and dry: Trade-off in arboreal calling in a treefrog mediated by local environment. Behavioral Ecology. 31. 10.1093/beheco/arz169.
Crawford EC, & Kampe, G. (1971). Physiological responses of the lizard Sauromalus obesus to changes in ambient temperature. American Journal of Physiology-Legacy Content, 220(5), 1256–1260. https://doi.org/10.1152/ajplegacy.1971.220.5.1256
Mautz, W. J. (1982). Correlation of both respiratory and cutaneous water losses of lizards with habitat aridity. Journal of Comparative Physiology ? B, 149(1), 25–30. https://doi.org/10.1007/bf00735711
Peterman, W. E., & Semlitsch, R. D. (2014). Spatial variation in water loss predicts terrestrial salamander distribution and population dynamics. Oecologia, 176(2), 357–369. https://doi.org/10.1007/s00442-014-3041-4
Phadnis, A., Manning, K. C., Schuett, G. W., & Rykaczewski, K. (2019). Role of scale wettability on rain-harvesting behavior in a desert-dwelling rattlesnake. ACS Omega, 4(25), 21141–21147. https://doi.org/10.1021/acsomega.9b02557
Sherbrooke, W. C. (1990). Rain-Harvesting in the Lizard, Phrynosoma cornutum: Behavior and Integumental Morphology. Journal of Herpetology, 24(3), 302. https://doi.org/10.2307/1564398
Thompson, G., & Withers, P. (1997). Patterns of gas exchange and extended non-ventilatory periods in small goannas (Squamata: Varanidae). Comparative Biochemistry and Physiology Part A: Physiology, 118(4), 1411–1417. https://doi.org/10.1016/s0300-9629(97)86807-7
Media Attributions
- Screenshot 2025-09-11 at 1.25.27 PM
- 4502 images.001
- rsif20150415f01
- Screenshot 2025-09-17 at 2.32.02 PM
- 19340700974_90630a5d0b_o
