8 Reproduction: Reptiles
To lay or not to lay.
In contrast to amphibians, amniotes have lost their larval stage and their embryonic development occurs primarily inside an egg. In non-avian reptiles, hatchlings emerge as smaller replicas of adults. A reptilian egg is thus provisioned with a full pantry fuelling the development of the embryo. The egg thus need enough room to fit this pantry so reptilian eggs are much larger relative to the mother’s body than amphibian eggs are. The pantry sustaining the development of amniotes is the yolk, one of four extra-embryonic membranes shared by all amniotes. The name amniotes comes from another extra-embryonic membrane, the amnion, which is a fluid filled sack cushioning the embryo.
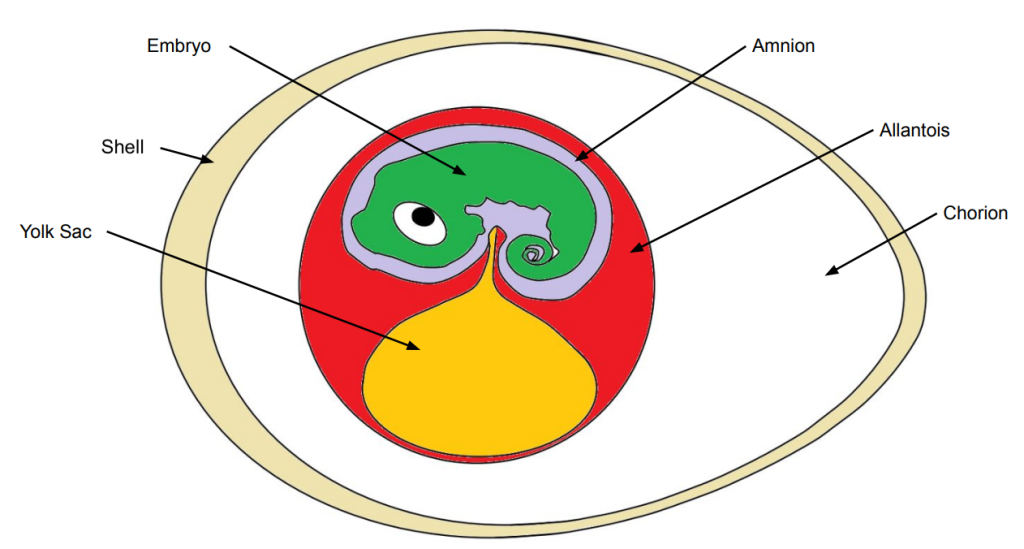
The eggshell is hard but porous allowing for the uptake of oxygen and the excretion of carbon dioxide. The eggshell is nonetheless far less permeable than the gelatinous layers surrounding the embryos of amphibians. The chorion, and the allantois, the other two extra-embryonic membranes are rich in blood vessels and move gases between the developing embryo and the inner surface of the shell where oxygen can diffuse in and carbon dioxide out. The allantois also doubles up as a waste storage compartment which ultimately develops into the urinary bladder and the cloaca.
The eggshell of reptiles can be soft and leathery or hard depending on the thickness of the outer mineral layers. Hard eggshells have sufficient water reserves for the entire development whereas soft shells absorb water from the environment.
Egg-laying, or oviparity, is the ancestral condition in amniotes. All crocodilians, tuataras, turtles, and birds, as well as about 80% of squamates (snakes and lizards) are oviparous. In oviparous species, embryos obtain all the energy and nutrients they need from the yolk which is produced during a process called vitellogenesis that occurs before ovulation (the release of eggs from the ovaries). This form of development in which all the provisioning occurs before ovulation and in which there is no nutritional exchanges between the mother and the embryo is called lecithotrophy.

About 20% of all livings squamates give birth to fully formed young and are thus considered live-bearers or viviparous. In viviparous species, the amount of provisioning the developing embryo receives from the mother varies among species. Some viviparous species are lecithotrophic like oviparous ones. This means that the female retains the eggs inside her oviduct for their entire development but no does not provide any nutrition during development. All the the nutrition comes from the yolk which was deposited before ovulation. In other words, these viviparous species retain eggs without a hard shell inside their bodies and there is no exchange between the mother and the embryo. In some species however, embryos receive nutrients from the mother after ovulation. This type of development is called matrotrophy and includes placentotrophy in which the exchange between the mother and the embryo occurs via the placenta (as in mammals). In reptiles, placentotrophy occurs in some species from the skink family (scincidae).
Viviparity evolved independently at least 100 times within squamates suggesting that it is advantageous in certain environments. The repeated evolution of viviparity also indicates that the transition between oviparity and viviparity is not a difficult one from an evolutionary point of view.
Several hypotheses have been proposed to explain the evolution of viviparity in squamates. A leading hypothesis suggests that viviparity is advantageous under cold climates because it gives females more control over the temperature of the developing embryos. Eggs in a nest cannot behaviourally thermoregulate but a gravid female can. This hypothesis stems from the observation that the frequency of viviparous squamates increases with latitude and altitude. A global analysis of viviparity indicates that embryos develop faster, and that offspring are more viable in viviparous species living at high latitudes compared to oviparous species at the same latitude. Moreover, stable and long‐lasting cold climatic conditions over the last 65 million years are correlated with transitions from oviparity to viviparity across squamates.

Fertilization
All reptiles have internal fertilization, and most species have an intromittent organ. Turtles and crocodilians have a single penis, while lizards and snakes have a penis split in two halves, each of which is called a hemipenis. Tuataras lack an intromittent organ, but fertilization is nonetheless internal in these reptiles. In tuataras sperm is transferred from a male to a female by cloacal apposition as in most birds.


Mating Season
As in amphibians, the reproductive season of reptiles is triggered by internal as well as environmental cues. In some species, sperm production (spermatogenesis), mating, and fertilization occurs at the same time. This type of reproductive cycle is called associated. In other species, each of these reproductive steps occur at different times. This type of reproductive cycle is called dissociated. Many turtle species living in temperate regions including in Ontario have a dissociated reproductive cycle. In these species mating occurs in fall and spring but eggs are only fertilized in the early summer. Females store viable sperm for several weeks in tubules located in their oviduct. In males, mating is also dissociated from spermatogenesis. Males produce sperm in the warmest months (summer) but mate in fall or spring. Between spermatogenesis and mating, males store their sperm in their epididymis where it remains viable for weeks.
Mating Systems
In temperate areas, reptiles usually mate in the spring, summer, and sometimes in the fall. The mating season of reptiles is more spread out than in amphibians and mating aggregations are less common than in amphibians. Therefore, scramble competition for mates appears less common in reptiles than in amphibians, at least in our area. Scrambles nonetheless occur in some species. Garter snakes and water snakes are a well known examples. Many snake species, including garter snakes, overwinter communally. Garter snakes mate immediately after they emerge from their hibernacula. Large aggregations of amorous snakes at communal hibernacula appear to set the stage for scramble competition for mates in which several males will court and attempt to mate with the same female simultaneously giving rise to what is often called mating balls. Northern water snakes also commonly form mating balls although they may not occur near hibernacula.

.

A similar situation occurs at least in some populations of the northern map turtle. At least at high latitudes, northern map turtles overwinter communally and mate just before and after hibernation. As in garter snakes and in water snakes, the mating system in this species appears to involve scrambles in which several males may attempt to mate with the same female either simultaneously or in short successions.

Scramble competition for mates also appear to occur in green sea turtles (Chelonia mydas). Females green turtles aggregate near nesting beaches during the nesting season and males compete for mates at these aggregations.

In species with scramble competition for mates, males tend to be smaller than females because larger body size in males does not increase mating opportunities. Females, on the other hand, always have an advantage in getting larger because reproductive outputs (e.g. clutch size or egg size) in females tend to increase with body size. The direction and the intensity of sexual size dimorphism in a species provide a clue about its mating system. In female-larger species, males do not tend to fight with one another nor do they tend to be territorial. This is the case for instance in garter snakes (Thamnophis sp), water snakes (Nerodia sp) and the northern map turtle. In male-larger species, males tend to fight to access mates and may be territorial. Of course there are many exceptions and multiple mating strategies may co-exist in one species.
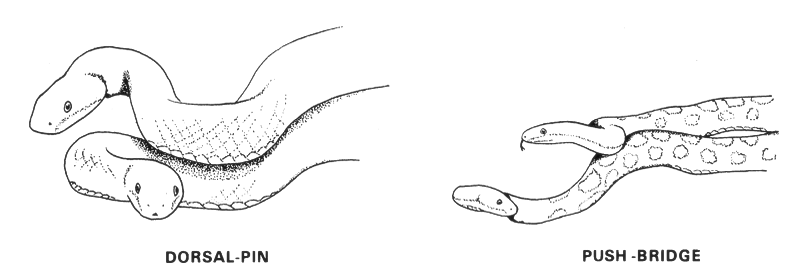
Some species have a mating system in which males fight with one another over females or territories. Gray rat snakes mate in May and June in Ontario, long after they have dispersed from their communal hibernacula. If two males encounter a female at the same time, they may engage in a dominance combat in which one male tries to push down the head of its rival using the upper part of its body; a manoeuvre called a dorsal-pin. The rival tries to counter the dorsal-pin with a counter-manoeuvre called the push-bridge. Eventually the sub-dominant male will abandon the fight. Consistent with the combat behaviour, male gray rat snakes are slightly larger than females suggesting a selective advantage for larger males.

In the common snapping turtle, males also fight with one another. Males are slightly larger than females in this species and more likely than females to have bite wounds on their head and neck indicating that males fight with one another either over territories or over mates.

In is unclear if male gray rat snakes and common snapping turtles actively maintain territories but territoriality is well documented in many lizards which can be more easily observed than snakes and turtles. Many species of lizards defend territories against competing males. An excellent example is the collared lizard (Crotaphytus collaris) from the southwestern United States. In this species, males fight over territories by biting each other. Males can inflict severe injuries on each other during territorial disputes and the strength of a male’s bite force correlates with the number of female in its territory as well as the number of offspring sired.

Female coercion may also play a role in mating. For instance, larger and older male painted turtles sport “teeth” called tomiodonts on their beaks as well as sharp serrations on their front marginal scutes. Both structures appear appear to be used to coerce females into mating and thus function as sexual weapons. Interestingly smaller and younger males in which the sexual weapons are less developed appear to rely more on courtship and female choice. Male painted turtles have elongated foreclaws which they use in a courtship display to woo females.


Coercion may also be used to a lesser extent by male northern map turtles. Males have been documented to bite female during the mating season. Moreover, during the mating season females, experience intense solicitation from males. For instance, more than half of the females observed swimming in one study were pursued by males. During the mating season, females reduce their activity and bury themselves in lake sediments presumably to hide from males.

Egg laying and parental care
Egg laying site varies between species of reptiles. Most species of turtles lay their eggs in a cavity in the ground and completely lack parental care. Oviparous snakes and lizards lay their eggs in a variety of substrates and habitats. Gray rat snakes for instance, lay their eggs in piles of decomposing organic matter which generate heat that accelerate the development of the eggs. Communal nesting, where multiple females lay their eggs in one location, is common in gray rat snakes. The advantages of communal nesting are unclear and may be related to the thermal quality of the nesting sites. If sites with good thermal quality for the development of the eggs are limited, communal nesting is expected.

Most species of reptiles lack parental care although some snakes and lizards show some parental. Crocodilians have the most elaborate parental care in reptiles. Females build nests and protect their nests and their young upon hatching. Parental care in crocodilians may have evolved because adults are well equipped to protect their nests against nest predators such as medium and large size mammals. Most parental care in reptiles take the form of eggs attendance as it is the case in the five lined skink (Plestiodon fasciatus).

Sex determination
Some species of reptiles have genotypic sex determination (GSD) with sex chromosomes. In some species, males are heterogametic (males are XY and females are XX as in mammals) while in other species, females are heretogametic sex (males are ZZ and females are ZW as in birds). GSD is found in snakes, some lizards and a few species of turtles (including the wood turtle). In most species of turtles, some lizards and, all crocodilians, sex is determined by temperature during the incubation period (temperature dependent sex determination or TSD). Different patterns of TSD exist in reptiles. In pattern IA, males are produced at low temperatures and females at high temperatures. This pattern is found in most turtles and in some squamates. Pattern IB is the reverse of IA with females being produced at low temperatures and males at high temperatures. This pattern is found in the Tuatara, and in some squamates. Finally in pattern II, males are produced at intermediate temperatures and females at the extremes. This pattern is found in a few species of turtles like the common snapping turtle (Chelydra serpentina), in some squamates and in all crocodilians. TSD has not yet been documented in a snake.

In TSD, temperature affects the expression of sex only before and during the development of the gonads which happens in the middle third of the developmental period. Thanks to work on turtles, the molecular underpinnings of TSD are now starting to be understood. In species with TSD, embryos have the potential to either become male or female. The temperature during a specific developmental window dictates which sex the embryo will turn into. For an embryo to become a male, a testis building gene called dmtt1 must be turned on. If dmtt1 is not turned on, ovaries develop instead of testis and the embryo becomes a female. Like all genes, dmtt1 has an on/off switch called a promotor region. For dmtt1 to be turned on, and thus for testis to be built, a special protein called a transcription activator must bind to its promoter region. A gene called kdm6b codes for that transcription activator. Researchers figured out the role of kdm6b in sex determination by experimentally knocking it off in turtles. Doing so produced females at temperatures that normally would have produced males. Temperature however does not affect the expression of kdm6b directly. Instead, it affects the expression of a third gene called stat3. stat3 encodes a transcription repressor (the opposite of a transcription activator) that switches off the expression of kdm6b. At female producing temperatures , stat3 is activated and turns off kdm6b so dmrt1 is not expressed and ovaries are built instead of testis. How temperature affects the expression of stat3 remains unclear but may depend on the amount of calcium inside the cell which is itself affected by temperature.

Phylogenetic analyses suggest that GSD is the ancestral state in vertebrates but that TSD is the ancestral state in reptiles. The implication here is that species with GSD reverted to the ancestral vertebrate state. This pattern raises interesting questions about the adaptive significance of TSD. Has TSD re-evolved several times because it is advantageous in certain environments? This important question remains largely unanswered but the leading hypothesis for the evolution of TSD posits that TSD can be advantageous in some environments. This hypothesis called the Charnov and Bull model (after the seminal paper by Eric Charnov and James Bull) suggests that TSD is advantageous when the fitness of males and females are optimized at different developmental temperatures. Empirically testing this model is however quite challenging because it is not possible to completely separate the effect of sex from the effect of incubation temperature on fitness. In other words, a stringent experimental test would require comparing the fitness of members or the same sex produced at temperatures that normally produced different sexes.
There are concerns that global warming is affecting the sex ratio of reptile populations with TSD. Some populations of sea turtles for instance have been reported to produce extremely biased sex ratio but it remains unclear if populations will adapt or to adjust their nesting behaviour to cope with the current rate of temperature increase.
References
Keevil, Matt & Hewitt, Brittainy & Brooks, Ronald & Litzgus, Jacqueline. (2017). Patterns of intraspecific aggression inferred from injuries in an aquatic turtle with male-biased size dimorphism. Canadian Journal of Zoology. 95. 10.1139/cjz-2016-0182.
Lappin, A Kristopher & Husak, Jerry. (2005). Weapon Performance, Not Size, Determines Mating Success and Potential Reproductive Output in the Collared Lizard (Crotaphytus collaris). The American naturalist. 166. 426-36. 10.1086/432564.
Lockley, Emma & Eizaguirre, Christophe. (2021). Effects of global warming on species with temperature‐dependent sex determination: Bridging the gap between empirical research and management. Evolutionary Applications. 14. 10.1111/eva.13226.
Ma, Liang & Buckley, Lauren & Huey, Raymond & Du, Wei-Guo. (2018). A global test of the cold-climate hypothesis for the evolution of viviparity of squamate reptiles. Global Ecology and Biogeography. 27. 10.1111/geb.12730.
Merchant-Larios, Horacio & Díaz-Hernández, Verónica & Cortez, Diego. (2021). Molecular and Cellular Mechanisms Underlying Temperature-Dependent Sex Determination in Turtles. Sexual Development. 15. 1-9. 10.1159/000515296.
Moldowan PD, RJ Brooks, and JD Litzgus. 2020. Demographics of injuries indicate sexual coercion in a population of Painted Turtle (Chrysemys picta). Canadian Journal of Zoology 98(4): 269–278.
Media Attributions
- file-86fef1ace23d4057a18928e83c9df9f7-image-17118726661661892155136_orig
- EM_2-23-18_3
- Reptiles mating-7
- Reptiles mating
- Screenshot 2025-10-15 at 10.02.17 AM
- Reptiles mating-5
- _H4A0004
- buried females-2
- TSD.001
