Oxidation-Reduction Reactions
Earth’s atmosphere contains about 20% molecular oxygen, O2, a chemically reactive gas that plays an essential role in the metabolism of aerobic organisms and in many environmental processes that shape the world. The term oxidation was originally used to describe chemical reactions involving O2, but its meaning has evolved to refer to a broad and important reaction class known as oxidation-reduction (redox) reactions. A few examples of such reactions will be used to develop a clear picture of this classification.
Some redox reactions involve the transfer of electrons between reactant species to yield ionic products, such as the reaction between sodium and chlorine to yield sodium chloride:
It is helpful to view the process with regard to each individual reactant, that is, to represent the fate of each reactant in the form of an equation called a half-reaction:
These equations show that Na atoms lose electrons while Cl atoms (in the Cl2 molecule) gain electrons, the “s” subscripts for the resulting ions signifying they are present in the form of a solid ionic compound. For redox reactions of this sort, the loss and gain of electrons define the complementary processes that occur:
In this reaction, then, sodium is oxidized and chlorine undergoes reduction. Viewed from a more active perspective, sodium functions as a reducing agent (reductant), since it provides electrons to (or reduces) chlorine. Likewise, chlorine functions as an oxidizing agent (oxidant), as it effectively removes electrons from (oxidizes) sodium.
Some redox processes, however, do not involve the transfer of electrons. Consider, for example, a reaction similar to the one yielding NaCl:
The product of this reaction is a covalent compound, so transfer of electrons in the explicit sense is not involved. To clarify the similarity of this reaction to the previous one and permit an unambiguous definition of redox reactions, a property called oxidation number has been defined. The oxidation number (or oxidation state) of an element in a compound is the charge its atoms would possess if the compound were ionic. The following guidelines are used to assign oxidation numbers to each element in a molecule or ion.
Assigning Oxidation Numbers
- The oxidation number of an atom in an elemental substance is zero.
- The oxidation number of a monatomic ion is equal to the ion’s charge.
- Oxidation numbers for common nonmetals are usually assigned as follows:
- Hydrogen: +1 when combined with nonmetals, −1 when combined with metals
- Oxygen: −2 in most compounds, sometimes −1 (so-called peroxides, O22−), very rarely −1/2 (so-called superoxides, O2−), positive values when combined with F (values vary)
- Halogens: −1 for F always, −1 for other halogens except when combined with oxygen or other halogens (positive oxidation numbers in these cases, varying values)
- The sum of oxidation numbers for all atoms in a molecule or polyatomic ion equals the charge on the molecule or ion.
Note: The proper convention for reporting charge is to write the number first, followed by the sign (e.g., 2+), while oxidation number is written with the reversed sequence, sign followed by number (e.g., +2). This convention aims to emphasize the distinction between these two related properties.
Example 18.1a
Assigning Oxidation Numbers
Follow the guidelines in this section of the text to assign oxidation numbers to all the elements in the following species:
- H2S
- SO32−
- Na2SO4
Solution
- According to guideline 3, the oxidation number for H is +1.
Using this oxidation number and the compound’s formula, guideline 4 may then be used to calculate the oxidation number for sulfur:
charge on H2S=0=(2 × +1) + (1 × x)x = 0−(2 × +1) = −2 -
Guideline 3 suggests the oxidation number for oxygen is −2.
Using this oxidation number and the ion’s formula, guideline 4 may then be used to calculate the oxidation number for sulfur:
charge on SO32− = −2 =(3 × −2) + (1 × x)
x = −2 − (3 × −2) = +4 -
For ionic compounds, it’s convenient to assign oxidation numbers for the cation and anion separately.
According to guideline 2, the oxidation number for sodium is +1.
Assuming the usual oxidation number for oxygen (−2 per guideline 3), the oxidation number for sulfur is calculated as directed by guideline 4:
charge on SO42− = −2 = (4 × −2) + (1 × x)
x = −2 − (4 × −2)= +6
Exercise 18.1a
Assign oxidation states to the elements whose atoms are underlined (the underlined elements are a. N, b. A, c. N, d. P) in each of the following compounds or ions:
- KNO3
- AlH3
- NH4+
- H2PO4−
Check Your Answers[1]
Using the oxidation number concept, an all-inclusive definition of redox reaction has been established. Oxidation-reduction (redox) reactions are those in which one or more elements involved undergo a change in oxidation number. (While the vast majority of redox reactions involve changes in oxidation number for two or more elements, a few interesting exceptions to this rule do exist as seen in Example 18.1b). Definitions for the complementary processes of this reaction class are correspondingly revised as shown here:
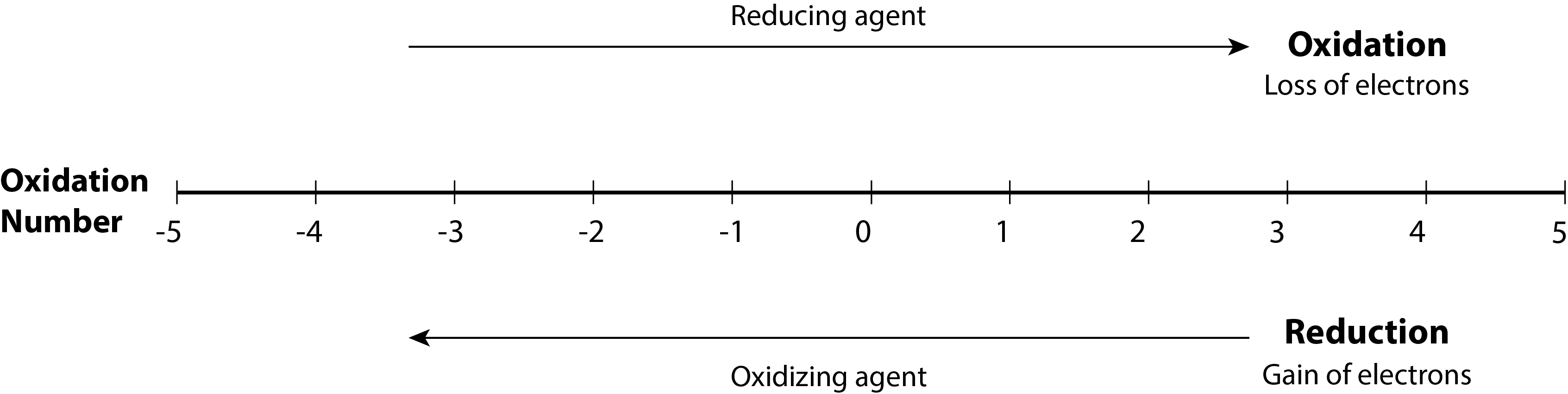
Returning to the reactions used to introduce this topic, they may now both be identified as redox processes. In the reaction between sodium and chlorine to yield sodium chloride, sodium is oxidized (its oxidation number increases from 0 in Na to +1 in NaCl) and chlorine is reduced (its oxidation number decreases from 0 in Cl2 to −1 in NaCl). In the reaction between molecular hydrogen and chlorine, hydrogen is oxidized (its oxidation number increases from 0 in H2 to +1 in HCl) and chlorine is reduced (its oxidation number decreases from 0 in Cl2 to −1 in HCl).
Several subclasses of redox reactions are recognized, including combustion reactions in which the reductant (also called a fuel) and oxidant (often, but not necessarily, molecular oxygen) react vigorously and produce significant amounts of heat, and often light, in the form of a flame. Solid rocket-fuel reactions are combustion processes. A typical propellant reaction in which solid aluminum is oxidized by ammonium perchlorate is represented by this equation:

